Dr Mark Trozzi(マーク・トロッツィ博士)が査読済み論文の約1,000件を掲載したサイトを作っています。
1000 peer reviewed articles on “Vaccine” injuries
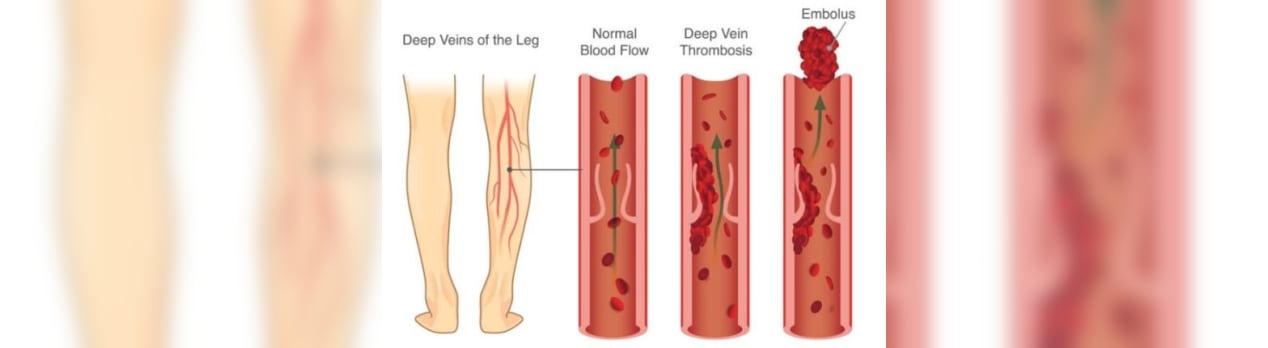
その中で2番目に多くの件数が報告されている血栓症の論文リストを転載させていただきます。
Thrombosis
血栓症、血栓塞栓症、血栓塞栓症。血栓症の原因には3つのカテゴリーがある:血管の損傷(カテーテルや手術)、血流の低下(不動)、および/または血栓症(血液自体が固まりやすい場合)。
論文総数 :150
Three cases of acute venous thromboembolism in women after vaccination against COVID-19 https://www.sciencedirect.com/science/article/pii/S2213333X21003929
Acute thrombosis of the coronary tree after vaccination against COVID-19 https://www.sciencedirect.com/science/article/abs/pii/S1936879821003988
US case reports of cerebral venous sinus thrombosis with thrombocytopenia after vaccination with Ad26.COV2.S (against covid-19), March 2 to April 21, 2020:
https://pubmed.ncbi.nlm.nih.gov/33929487/
Portal vein thrombosis associated with ChAdOx1 nCov-19 vaccine: https://www.thelancet.com/journals/langas/article/PIIS2468-1253(21)00197-7/
Management of cerebral and splanchnic vein thrombosis associated with thrombocytopenia in subjects previously vaccinated with Vaxzevria (AstraZeneca): position statement of the Italian Society for the Study of Hemostasis and Thrombosis (SISET):
https://pubmed.ncbi.nlm.nih.gov/33871350/
Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines: https://www.sciencedirect.com/science/article/abs/pii/S0735675721004381
Covid-19 vaccine-induced thrombosis and thrombocytopenia: a commentary on an important and practical clinical dilemma:
https://www.sciencedirect.com/science/article/abs/pii/S0033062021000505
Thrombosis with thrombocytopenia syndrome associated with COVID-19 viral vector vaccines: https://www.sciencedirect.com/science/article/abs/pii/S0953620521001904
COVID-19 vaccine-induced immune-immune thrombotic thrombocytopenia: an emerging cause of splanchnic vein thrombosis:
https://www.sciencedirect.com/science/article/pii/S1665268121000557
The roles of platelets in COVID-19-associated coagulopathy and vaccine-induced immune thrombotic immune thrombocytopenia (covid): https://www.sciencedirect.com/science/article/pii/S1050173821000967
Roots of autoimmunity of thrombotic events after COVID-19 vaccination: https://www.sciencedirect.com/science/article/abs/pii/S1568997221002160
Thrombotic immune thrombocytopenia induced by SARS-CoV-2 vaccine: https://www.nejm.org/doi/full/10.1056/nejme2106315
Thrombosis and thrombocytopenia after vaccination with ChAdOx1 nCoV-19 https://www.nejm.org/doi/full/10.1056/NEJMoa2104882?query=recirc_curatedRelated_article
Thrombotic thrombocytopenia after vaccination with ChAdOx1 nCov-19 https://www.nejm.org/doi/full/10.1056/NEJMoa2104840?query=recirc_curatedRelated_article
Post-mortemfindings in vaccine-induced thrombotic thrombocytopenia (covid-19): https://haematologica.org/article/view/haematol.2021.279075
Comparison of vaccine-induced thrombotic episodes between ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines:
https://www.sciencedirect.com/science/article/abs/pii/S0896841121000895
Hypothesis behind the very rare cases of thrombosis with thrombocytopenia syndrome after SARS-CoV-2 vaccination:
https://www.sciencedirect.com/science/article/abs/pii/S0049384821003315
Primary adrenal insuficiency associated with thrombotic immune thrombocytopenia induced by the Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine (VITT): https://www.sciencedirect.com/science/article/pii/S0953620521002363
“Portal vein thrombosis occurring after thefirst dose of SARS-CoV-2 mRNA vaccine in a patient with antiphospholipid syndrome”:
https://www.sciencedirect.com/science/article/pii/S2666572721000389
Early results of bivalirudin treatment for thrombotic thrombocytopenia and cerebral venous sinus thrombosis after vaccination with Ad26.COV2.S: https://www.sciencedirect.com/science/article/pii/S0196064421003425
Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection: https://www.sciencedirect.com/science/article/abs/pii/S0896841121000706
Prothrombotic immune thrombocytopenia after COVID-19 vaccination: https://www.sciencedirect.com/science/article/pii/S0006497121009411
Vaccine-induced thrombotic thrombocytopenia: the dark chapter of a success story: https://www.sciencedirect.com/science/article/pii/S2589936821000256
Thrombosis after COVID-19 vaccination: possible link to ACE pathways: https://www.sciencedirect.com/science/article/pii/S0049384821004369
Vaccine-induced thrombotic thrombocytopenia, a rare but severe case of friendlyfiwre in the battle against the COVID-19 pandemic: What pathogenesis?: https://www.sciencedirect.com/science/article/pii/S0953620521002314
Thrombocytopenia and intracranial venous sinus thrombosis after exposure to the “AstraZeneca COVID-19 vaccine”:
https://pubmed.ncbi.nlm.nih.gov/33918932/
Thrombosis with thrombocytopenia after messenger RNA vaccine -1273: https://pubmed.ncbi.nlm.nih.gov/34181446/
First dose of ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic, and hemorrhagic events in Scotland:
https://www.nature.com/articles/s41591-021-01408-4
PF4 immunoassays in vaccine-induced thrombotic thrombocytopenia: https://www.nejm.org/doi/full/10.1056/NEJMc2106383
Antibody epitopes in vaccine-induced immune immune thrombotic thrombocytopenia: https://www.nature.com/articles/s41586-021-03744-4
Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines:. https://www.sciencedirect.com/science/article/abs/pii/S0735675721004381
.
Immune thrombosis and thrombocytopenia (VITT) associated with the COVID-19 vaccine: diagnostic and therapeutic recommendations for a new syndrome:
https://pubmed.ncbi.nlm.nih.gov/33987882/
Laboratory testing for suspicion of COVID-19 vaccine-induced thrombotic (immune) thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34138513/
Intracerebral haemorrhage due to thrombosis with thrombocytopenia syndrome after COVID-19 vaccination: thefirst fatal case in Korea:
https://pubmed.ncbi.nlm.nih.gov/34402235/
Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and positive SARS-CoV-2 tests: self-controlled case series study:
https://pubmed.ncbi.nlm.nih.gov/34446426/
Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis after covid-19 vaccination; a systematic review:
https://pubmed.ncbi.nlm.nih.gov/34365148/
.
Primary adrenal insuficiency associated with thrombotic immune thrombocytopenia induced by Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine (VITT):
https://pubmed.ncbi.nlm.nih.gov/34256983/
Thromboaspiration infusion andfibrinolysis for portomesenteric thrombosis after administration of AstraZeneca COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34132839/
59-year-old woman with extensive deep venous thrombosis and pulmonary thromboembolism 7 days after a first dose of Pfizer-BioNTech BNT162b2 mRNA vaccine COVID-19: https://pubmed.ncbi.nlm.nih.gov/34117206/
Thrombosis with thrombocytopenia syndrome (TTS) following AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 vaccination: risk-benefit analysis for persons <60 years in Australia: https://pubmed.ncbi.nlm.nih.gov/34272095/
Comparison of vaccine-induced thrombotic events between ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines:
https://pubmed.ncbi.nlm.nih.gov/34139631/
.
Bilateral superior ophthalmic vein thrombosis, ischemic stroke and immune thrombocytopenia after vaccination with ChAdOx1 nCoV-19:
https://pubmed.ncbi.nlm.nih.gov/33864750/
celiac artery and splenic artery thrombosis complicated by splenic infarction 7 days after the? rst dose of Oxford vaccine, causal relationship or coincidence:
https://pubmed.ncbi.nlm.nih.gov/34261633/
.
Primary adrenal insuficiency associated with Oxford-AstraZeneca ChAdOx1 nCoV-19 (VITT) vaccine-induced immune thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34256983/
Thrombosis with thrombocytopenia syndrome after COVID-19 immunization: https://pubmed.ncbi.nlm.nih.gov/34236343/
Thrombosis with thrombocytopenia syndrome associated with COVID-19 viral vector vaccines: https://pubmed.ncbi.nlm.nih.gov/34092488/
Thromboaspiration infusion andfibrinolysis for portomesenteric thrombosis after administration of the AstraZeneca COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34132839/
.
Atypical thrombosis associated with the vaccine VaxZevriaR (AstraZeneca): data from the French network of regional pharmacovigilance centers:
https://pubmed.ncbi.nlm.nih.gov/34083026/
.
Vaccine-induced thrombosis and thrombocytopenia with bilateral adrenal haemorrhage: https://pubmed.ncbi.nlm.nih.gov/34235757/.
Palmar digital vein thrombosis after Oxford-AstraZeneca COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34473841/
.
Cutaneous thrombosis associated with cutaneous necrosis following Oxford-AstraZeneca COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34189756/
Thrombosis with thrombocytopenia after Messenger vaccine RNA-1273: https://pubmed.ncbi.nlm.nih.gov/34181446/
Coronavirus (COVID-19) Vaccine-induced immune thrombotic thrombocytopenia (VITT): https://pubmed.ncbi.nlm.nih.gov/34033367/
Comparison of adverse drug reactions among four COVID-19 vaccines in Europe using the EudraVigilance database: Thrombosis in unusual sites:
https://pubmed.ncbi.nlm.nih.gov/34375510/
Immunoglobulin adjuvant for vaccine-induced immune thrombotic thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34107198/
Severe vaccine-induced thrombotic thrombocytopenia following vaccination with COVID-19: an autopsy case report and review of the literature:
https://pubmed.ncbi.nlm.nih.gov/34355379/
.
Platelet activation and modulation in thrombosis with thrombocytopenia syndrome associated with the ChAdO × 1 nCov-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34474550/
Report of the International Cerebral Venous Thrombosis Consortium on cerebral venous thrombosis after SARS-CoV-2 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34462996/
Immune thrombocytopenia associated with the Pfizer-BioNTech COVID-19 mRNA vaccine BNT162b2: https://www.sciencedirect.com/science/article/pii/S2214250921002018
Secondary immune thrombocytopenia putatively attributable to COVID-19 vaccination: https://casereports.bmj.com/content/14/5/e242220.abstract.
Immune thrombocytopenia following Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34155844/
Newly diagnosed idiopathic thrombocytopenia after COVID-19 vaccine administration: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8176657/
Idiopathic thrombocytopenic purpura and the Modern Covid-19 vaccine: https://www.annemergmed.com/article/S0196-0644(21)00122-0/fulltext.
Thrombocytopenia after Pfizer and Moderna SARS vaccination ? CoV -2:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8014568/
Immune thrombocytopenic purpura and acute liver injury after COVID-19 vaccination: https://casereports.bmj.com/content/14/7/e242678
Carotid artery immune thrombosis induced by adenovirus-vectored COVID-19 vaccine: case report: https://pubmed.ncbi.nlm.nih.gov/34312301/
.
The roles of platelets in COVID-19-associated coagulopathy and vaccine-induced immune-immune thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34455073/
Cerebral venous sinus thrombosis negative for anti-PF4 antibody without thrombocytopenia after immunization with COVID-19 vaccine in a non-comorbid elderly Indian male treated with conventional heparin-warfarin-based anticoagulation:
https://pubmed.ncbi.nlm.nih.gov/34186376/
Arterial events, venous thromboembolism, thrombocytopenia and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population-based cohort study: https://pubmed.ncbi.nlm.nih.gov/33952445/
Procoagulant microparticles: a possible link between vaccine-induced immune thrombocytopenia (VITT) and cerebral sinus venous thrombosis:
https://pubmed.ncbi.nlm.nih.gov/34129181/
S. case reports of cerebral venous sinus thrombosis with thrombocytopenia after vaccination with Ad26.COV2.S, March 2-April 21, 2021:
https://pubmed.ncbi.nlm.nih.gov/33929487/
.
Malignant cerebral infarction after vaccination with ChAdOx1 nCov-19: a catastrophic variant of vaccine-induced immune-mediated thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34341358/
Acute ischemic stroke revealing immune thrombotic thrombocytopenia induced by ChAdOx1 nCov-19 vaccine: impact on recanalization strategy:
https://pubmed.ncbi.nlm.nih.gov/34175640/
Vaccine-induced immune thrombotic immune thrombocytopenia (VITT): a new clinicopathologic entity with heterogeneous clinical presentations:
https://pubmed.ncbi.nlm.nih.gov/34159588/
.
Imaging and hematologicfindings in thrombosis and thrombocytopenia after vaccination with ChAdOx1 nCoV-19 (AstraZeneca):
https://pubmed.ncbi.nlm.nih.gov/34402666/
Autoimmunity roots of thrombotic events after vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34508917/
Cerebral venous sinus thrombosis after vaccination: the UK experience: https://pubmed.ncbi.nlm.nih.gov/34370974/
Cutaneous thrombosis associated with cutaneous necrosis following Oxford-AstraZeneca COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34189756/
Myocardial infarction and azygos vein thrombosis after vaccination with ChAdOx1 nCoV-19 in a hemodialysis patient:
https://pubmed.ncbi.nlm.nih.gov/34650896/
Refractory vaccine-induced immune thrombotic thrombocytopenia (VITT) treated with delayed therapeutic plasma exchange (TPE):
https://pubmed.ncbi.nlm.nih.gov/34672380/
.
Rare case of COVID-19 vaccine-associated intracranial hemorrhage with venous sinus thrombosis: https://pubmed.ncbi.nlm.nih.gov/34556531/
.
Delayed headache after COVID-19 vaccination: a warning sign for vaccine-induced cerebral venous thrombosis:
https://pubmed.ncbi.nlm.nih.gov/34535076/
.
Clinical features of vaccine-induced thrombocytopenia and immune thrombosis: https://pubmed.ncbi.nlm.nih.gov/34379914/
.
Predictors of mortality in thrombotic thrombocytopenia after adenoviral COVID-19 vaccination: the FAPIC score:
https://pubmed.ncbi.nlm.nih.gov/34545400/
Ischemic stroke as a presenting feature of immune thrombotic thrombocytopenia induced by ChAdOx1-nCoV-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34035134/
Endovascular treatment for vaccine-induced cerebral venous sinus thrombosis and thrombocytopenia after vaccination with ChAdOx1 nCoV-19: report of three cases: https://pubmed.ncbi.nlm.nih.gov/34782400/
Possible triggers of thrombocytopenia and/or hemorrhage by BNT162b2 vaccine, P? zer-BioNTech: https://pubmed.ncbi.nlm.nih.gov/34660652/
.
Multiple sites of arterial thrombosis in a 35-year-old patient after vaccination with ChAdOx1 (AstraZeneca), which required emergency femoral and carotid surgical thrombectomy: https://pubmed.ncbi.nlm.nih.gov/34644642/
Case series of vaccine-induced thrombotic thrombocytopenia in a London teaching hospital: https://pubmed.ncbi.nlm.nih.gov/34694650/
Neuro-ophthalmic complications with thrombocytopenia and thrombosis induced by ChAdOx1 nCoV-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34726934/
Thrombotic events after COVID-19 vaccination in over 50 years of age: results of a population-based study in Italy:
https://pubmed.ncbi.nlm.nih.gov/34835237/
Intracerebral hemorrhage associated with vaccine-induced thrombotic thrombocytopenia after ChAdOx1 nCOVID-19 vaccination in a pregnant woman:
https://pubmed.ncbi.nlm.nih.gov/34261297/
Age- and sex-specific incidence of cerebral venous sinus thrombosis associated with Ad26.COV2.S COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34724036/
.
Genital necrosis with cutaneous thrombosis following vaccination with COVID-19 mRNA: https://pubmed.ncbi.nlm.nih.gov/34839563/
Cerebral venous sinus thrombosis after mRNA-based COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34783932/
.
COVID-19 vaccine-induced immune thrombosis with thrombocytopenia thrombosis (VITT) and shades of gray in thrombus formation:
https://pubmed.ncbi.nlm.nih.gov/34624910/
Acute ST-segment elevation myocardial infarction secondary to vaccine-induced immune thrombosis with thrombocytopenia (VITT):
https://pubmed.ncbi.nlm.nih.gov/34580132/
Thrombosis with thrombocytopenia syndrome (TTS) after vaccination with AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19: a risk-bene? t analysis for persons <60% risk-benefet analysis for people <60 years in Australia:
https://pubmed.ncbi.nlm.nih.gov/34272095/
Characteristics and outcomes of patients with cerebral venous sinus thrombosis in thrombotic immune thrombocytopenia induced by SARS-CoV-2 vaccine: https://jamanetwork.com/journals/jamaneurology/fullarticle/2784622
Case study of thrombosis and thrombocytopenia syndrome after administration of the AstraZeneca COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34781321/
Thrombosis with Thrombocytopenia Syndrome Associated with COVID-19 Vaccines: https://pubmed.ncbi.nlm.nih.gov/34062319/
Cerebral venous sinus thrombosis following vaccination with ChAdOx1: thefirst case of definite thrombosis with thrombocytopenia syndrome in India:
https://pubmed.ncbi.nlm.nih.gov/34706921/
COVID-19 vaccine-associated thrombosis with thrombocytopenia syndrome (TTS): systematic review and post hoc analysis:
https://pubmed.ncbi.nlm.nih.gov/34698582/
Concerns for adverse effects of thrombocytopenia and thrombosis after adenovirus-vectored COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34541935/
Cerebral venous sinus thrombosis after COVID-19 vaccination: neurologic and radiologic management: https://pubmed.ncbi.nlm.nih.gov/34327553/
.
Case report: cerebral sinus vein thrombosis in two patients with AstraZeneca SARS-CoV-2 vaccine: https://pubmed.ncbi.nlm.nih.gov/34609603/
Vaccine-induced immune thrombosis and thrombocytopenia syndrome after adenovirus-vectored severe acute respiratory syndrome coronavirus 2 vaccination: a new hypothesis on mechanisms and implications for future vaccine development:
https://pubmed.ncbi.nlm.nih.gov/34664303/
.
Thrombosis in peripheral artery disease and thrombotic thrombocytopenia following adenoviral COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34649281/
Cerebral venous sinus thrombosis and thrombotic events after vector-based COVID-19 vaccines: systematic review and meta-analysis:
https://pubmed.ncbi.nlm.nih.gov/34610990/
.
Thrombosis after COVID-19 vaccination: possible link to ACE pathways: https://pubmed.ncbi.nlm.nih.gov/34479129/
Major artery thrombosis and vaccination against ChAdOx1 nCov-19 https://pubmed.ncbi.nlm.nih.gov/34839830/
Understanding the risk of thrombosis with thrombocytopenia syndrome following Ad26.COV2.S vaccination:
https://pubmed.ncbi.nlm.nih.gov/34595694/