画像類似が認められる論文#2
Shibasaki Y, Matsubara H (corresponding author), Nozawa Y, Mori Y, Masaki H, Kosaki A, Tsutsumi Y, Uchiyama Y, Fujiyama S, Nose A, Iba O, Tateishi E, Hasegawa T, Horiuchi M, Nahmias C, Iwasaka T.
Angiotensin II type 2 receptor inhibits epidermal growth factor receptor transactivation by increasing association of SHP-1 tyrosine phosphatase
Hypertension. 2001 Sep;38(3):367-72.
Department of Medicine II, Kansai Medical University, Moriguchi, Osaka, Japan.
責任著者 : 松原弘明、 筆頭著者 : 柴崎泰延
松原弘明氏の論文発表当時の所属 : 関西医科大学第二内科
画像類似指摘項目No.1
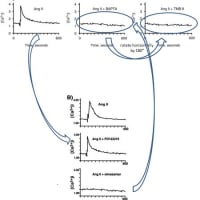
・論文#1 (Kidney Int. 2001;60:2153-63.)のFig.2Bの「Ang II + BAPTA」のグラフを上下反転させたものが、その右隣りの「AngII + TMB 8」のグラフと類似しています。
・また、論文#1のFig.2Bの「Ang II」のグラフが、論文#2 (Hypertension. 2001;38:367-72.の「AngII + PD123319」のグラフと類似しています。
・さらに、論文#1のFig.2Bの「Ang II + BAPTA」のグラフが、論文#2のFig.2Bの「AngII + Olmesartan」のグラフと類似しています。
画像類似指摘項目No.3
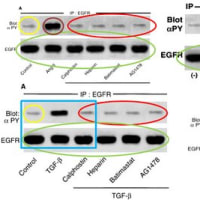
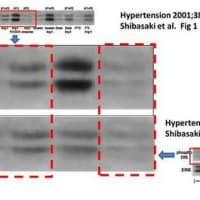
・論文#1のFig.1Aの「BlotαPY」の「Control」のバンド画像は、論文#4 (Kidney Int. 2002;62:799-808.)のFig.5Aの「BlotαPY」の「Control」のバンド画像と類似しています。
・また、論文#1のFig.1Aの「EGFR」の6レーン分のバンド画像は、論文#2のFig.2Aの「EGFR」の6レーン分のバンド画像や、論文#4のFig.5Aの「EGFR」の6レーン分のバンド画像や、論文#3 (Circ Res. 2001;88:22-9.)のFig.4の「EGFR」(下側のパネル)の左側6レーン分のバンド画像((-), AII, PD123319, PD123319 CS866, Banadate, Vanadate CS866 の6レーン)と、類似しています。
・また、論文#1のFig.1Aの「BlotαPY」の右側4レーン分の画像(AngII: Calphostin, Heparin, Batimastat,AG1478の4レーン)は、論文#4のFig.5Aの「BlotαPY」の右側4レーン分の画像(TGF-β: Calphostin, Heparin, Batimastat,AG1478の4レーン)と類似しています。
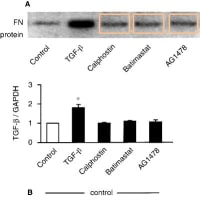
・また、論文#1のFig.6Cの左側の図の「BlotαPYの3レーン分の画像と「EGFR」の3レーン分の画像は、それぞれ、論文#4のFig.5Aの「BlotαPY」の左側3レーン分の画像(Control, TGF-β, Calphostin)と「EGFR」の左側3レーン分の画像(Control, TGF-β, Calphostin)と、類似しています。
画像類似指摘項目No.6
論文#2のFig.1Bの「Phospho ERK」の「(-)」のバンド画像と「Ang II」のバンド画像と「Ang II Olmesartan」のバンド画像は、それぞれ、同論文#2のFig.3Cの一番右側の図の「Phospho ERK」の「(-)」のバンド画像と「Ang II」のバンド画像と「Ang II Olmesartan」のバンド画像に、類似しています。
画像類似指摘項目No.7
論文#2のFig.2Aの「EGFR」の「Ang II」, 「Ang II PD123319」, 「Ang II vanadate」, 「Ang II olmesartan」の中央4レーン分のバンド画像は、同論文#2のFig3Bの「EGFR」の「(-)」, 「Ang II」, 「Ang II olmesartan」, 「Ang II PD123319」の4レーン分のバンド画像と類似しています。
画像類似指摘項目No.9
・論文#5のFig.2Bの二つのグラフは、論文#2のFig.2Bの二つのグラフに類似しています。
・また、論文#5のTable 1と、論文#2 のTable 1が類似しています。
・また、論文#5のFig.4の「GAPDH」の画像と、論文#2のFig.4の「GAPDH」の画像が類似しています。
・また、論文#2と論文#5には、下記のように類似文章が多数認められます。
BBRC These differences might be due to variation of cell types or reflect the complexity of the network involved in negative regulation of ERK activity. Thus, further dissection of the AT2 signaling pathway and identification of cross-point with Pyk2-JNK intracellular cascade, may provide new perspectives for pharmacological targeting of proliferative diseases.
Hypertension These differences might be due to a variation of cell types or might reflect the complexity of the network involved in negative regulation of ERK activity. Thus, further dissection of the AT2 signaling pathway and identification of the cross-point with EGFR cascades may provide new perspectives for pharmacological targeting of proliferative diseases and a unique example of negative cross-talk in growth signals.
BBRC However, the molecules interacting with SHP-1 were not defined in these earlier studies. Interestingly, Li et al. reported that platelet thrombin receptor causes SHP-1 tyrosine-phosphorylation in a PTX dependent manner, and suggested the role of tyrosine kinases linked to the thrombin receptor by Gi-protein.
Hypertension However, the molecules interacting with SHP-1 were not defined in these earlier studies. Li et al reported that platelet thrombin receptor causes SHP-1 tyrosine phosphorylation in a PTX-dependent manner and suggested the role of tyrosine kinases linked to the thrombin receptor by Gi protein.
BBRC Recently, a structural model for SHP-1 was proposed, in which SH2 domains of SHP-1 were shown to be capable of interacting with its C terminus in a phosphotyrosine-dependent manner and thereby drive the PTPase domain in an inactive conformation.
Hypertension Recently, a structural model for SHP-1 was proposed in which SH2 domains of SHP-1 were shown to be capable of interacting with its C terminus in a phosphotyrosine-dependent manner and thereby drive the PTPase domain in an inactive conformation.
BBRC It is possible that the conformational change of SHP-1 induced by AT2 causes an increased association of SHP-1 with downstream molecules of Pyk2, leading to JNK inactivation. Thus, we speculated that AT2 has the capacity to disrupt this intramolecular interaction.
Hypertension It is possible that the conformational change of SHP-1 induced by AT2 leads to the increased association of SHP-1 with EGFR and forms the basis for activation toward the receptor as observed in our study. Thus, AT2 may have the capacity to disrupt this intramolecular interaction.
BBRC SHP-1 is predominantly expressed in hematopoietic cells and plays a key role in hematopoiesis. Although the role of SHP-1 in VSMC has not been defined in detail, the present study suggested a novel function of SHP-1 in AT2-mediated JNK inactivation followed by a growth inhibitory action.
Hypertension SHP-1 is predominantly expressed in hematopoietic cells and plays a key role in hematopoiesis. Although the role of SHP-1 in VSMCs has not been defined in detail, the present study suggested a novel function of SHP-1 in AT2-mediated ERGFRinactivation followed by a growth inhibitory action.
BBRC Since SHP-1 was reported to interact with SHP-2, further studies are required to define the relationship between SHP-1 and SHP-2 in the mechanism for AT2 activation.
Hypertension Because SHP-1 was reported to interact with SHP-2, further studies are required to define the relationship between SHP-1 and SHP-2 in the mechanism of
Shibasaki Y, Matsubara H (corresponding author), Nozawa Y, Mori Y, Masaki H, Kosaki A, Tsutsumi Y, Uchiyama Y, Fujiyama S, Nose A, Iba O, Tateishi E, Hasegawa T, Horiuchi M, Nahmias C, Iwasaka T.
Angiotensin II type 2 receptor inhibits epidermal growth factor receptor transactivation by increasing association of SHP-1 tyrosine phosphatase
Hypertension. 2001 Sep;38(3):367-72.
Department of Medicine II, Kansai Medical University, Moriguchi, Osaka, Japan.
責任著者 : 松原弘明、 筆頭著者 : 柴崎泰延
松原弘明氏の論文発表当時の所属 : 関西医科大学第二内科
画像類似指摘項目No.1
・論文#1 (Kidney Int. 2001;60:2153-63.)のFig.2Bの「Ang II + BAPTA」のグラフを上下反転させたものが、その右隣りの「AngII + TMB 8」のグラフと類似しています。
・また、論文#1のFig.2Bの「Ang II」のグラフが、論文#2 (Hypertension. 2001;38:367-72.の「AngII + PD123319」のグラフと類似しています。
・さらに、論文#1のFig.2Bの「Ang II + BAPTA」のグラフが、論文#2のFig.2Bの「AngII + Olmesartan」のグラフと類似しています。
画像類似指摘項目No.3
・論文#1のFig.1Aの「BlotαPY」の「Control」のバンド画像は、論文#4 (Kidney Int. 2002;62:799-808.)のFig.5Aの「BlotαPY」の「Control」のバンド画像と類似しています。
・また、論文#1のFig.1Aの「EGFR」の6レーン分のバンド画像は、論文#2のFig.2Aの「EGFR」の6レーン分のバンド画像や、論文#4のFig.5Aの「EGFR」の6レーン分のバンド画像や、論文#3 (Circ Res. 2001;88:22-9.)のFig.4の「EGFR」(下側のパネル)の左側6レーン分のバンド画像((-), AII, PD123319, PD123319 CS866, Banadate, Vanadate CS866 の6レーン)と、類似しています。
・また、論文#1のFig.1Aの「BlotαPY」の右側4レーン分の画像(AngII: Calphostin, Heparin, Batimastat,AG1478の4レーン)は、論文#4のFig.5Aの「BlotαPY」の右側4レーン分の画像(TGF-β: Calphostin, Heparin, Batimastat,AG1478の4レーン)と類似しています。
・また、論文#1のFig.6Cの左側の図の「BlotαPYの3レーン分の画像と「EGFR」の3レーン分の画像は、それぞれ、論文#4のFig.5Aの「BlotαPY」の左側3レーン分の画像(Control, TGF-β, Calphostin)と「EGFR」の左側3レーン分の画像(Control, TGF-β, Calphostin)と、類似しています。
画像類似指摘項目No.6
論文#2のFig.1Bの「Phospho ERK」の「(-)」のバンド画像と「Ang II」のバンド画像と「Ang II Olmesartan」のバンド画像は、それぞれ、同論文#2のFig.3Cの一番右側の図の「Phospho ERK」の「(-)」のバンド画像と「Ang II」のバンド画像と「Ang II Olmesartan」のバンド画像に、類似しています。
画像類似指摘項目No.7
論文#2のFig.2Aの「EGFR」の「Ang II」, 「Ang II PD123319」, 「Ang II vanadate」, 「Ang II olmesartan」の中央4レーン分のバンド画像は、同論文#2のFig3Bの「EGFR」の「(-)」, 「Ang II」, 「Ang II olmesartan」, 「Ang II PD123319」の4レーン分のバンド画像と類似しています。
画像類似指摘項目No.9
・論文#5のFig.2Bの二つのグラフは、論文#2のFig.2Bの二つのグラフに類似しています。
・また、論文#5のTable 1と、論文#2 のTable 1が類似しています。
・また、論文#5のFig.4の「GAPDH」の画像と、論文#2のFig.4の「GAPDH」の画像が類似しています。
・また、論文#2と論文#5には、下記のように類似文章が多数認められます。
BBRC These differences might be due to variation of cell types or reflect the complexity of the network involved in negative regulation of ERK activity. Thus, further dissection of the AT2 signaling pathway and identification of cross-point with Pyk2-JNK intracellular cascade, may provide new perspectives for pharmacological targeting of proliferative diseases.
Hypertension These differences might be due to a variation of cell types or might reflect the complexity of the network involved in negative regulation of ERK activity. Thus, further dissection of the AT2 signaling pathway and identification of the cross-point with EGFR cascades may provide new perspectives for pharmacological targeting of proliferative diseases and a unique example of negative cross-talk in growth signals.
BBRC However, the molecules interacting with SHP-1 were not defined in these earlier studies. Interestingly, Li et al. reported that platelet thrombin receptor causes SHP-1 tyrosine-phosphorylation in a PTX dependent manner, and suggested the role of tyrosine kinases linked to the thrombin receptor by Gi-protein.
Hypertension However, the molecules interacting with SHP-1 were not defined in these earlier studies. Li et al reported that platelet thrombin receptor causes SHP-1 tyrosine phosphorylation in a PTX-dependent manner and suggested the role of tyrosine kinases linked to the thrombin receptor by Gi protein.
BBRC Recently, a structural model for SHP-1 was proposed, in which SH2 domains of SHP-1 were shown to be capable of interacting with its C terminus in a phosphotyrosine-dependent manner and thereby drive the PTPase domain in an inactive conformation.
Hypertension Recently, a structural model for SHP-1 was proposed in which SH2 domains of SHP-1 were shown to be capable of interacting with its C terminus in a phosphotyrosine-dependent manner and thereby drive the PTPase domain in an inactive conformation.
BBRC It is possible that the conformational change of SHP-1 induced by AT2 causes an increased association of SHP-1 with downstream molecules of Pyk2, leading to JNK inactivation. Thus, we speculated that AT2 has the capacity to disrupt this intramolecular interaction.
Hypertension It is possible that the conformational change of SHP-1 induced by AT2 leads to the increased association of SHP-1 with EGFR and forms the basis for activation toward the receptor as observed in our study. Thus, AT2 may have the capacity to disrupt this intramolecular interaction.
BBRC SHP-1 is predominantly expressed in hematopoietic cells and plays a key role in hematopoiesis. Although the role of SHP-1 in VSMC has not been defined in detail, the present study suggested a novel function of SHP-1 in AT2-mediated JNK inactivation followed by a growth inhibitory action.
Hypertension SHP-1 is predominantly expressed in hematopoietic cells and plays a key role in hematopoiesis. Although the role of SHP-1 in VSMCs has not been defined in detail, the present study suggested a novel function of SHP-1 in AT2-mediated ERGFRinactivation followed by a growth inhibitory action.
BBRC Since SHP-1 was reported to interact with SHP-2, further studies are required to define the relationship between SHP-1 and SHP-2 in the mechanism for AT2 activation.
Hypertension Because SHP-1 was reported to interact with SHP-2, further studies are required to define the relationship between SHP-1 and SHP-2 in the mechanism of











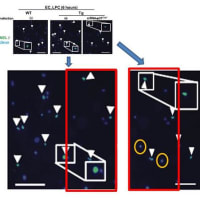
![画像類似の論文#16 (Kidney Int. 2011 Dec 7. doi: 10.1038/ki.2011.403. [Epub ahead of print])](https://blogimg.goo.ne.jp/image/upload/f_auto,q_auto,t_image_square_m/v1/user_image/21/de/685e525c47bce551c122291973de56dd.jpg)
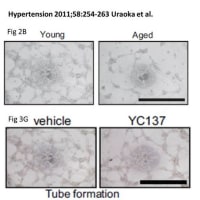
![画像類似の論文#16 (Kidney Int. 2011 Dec 7. doi: 10.1038/ki.2011.403. [Epub ahead of print])](https://blogimg.goo.ne.jp/image/upload/f_auto,q_auto,t_image_square_m/v1/user_image/57/ac/4bed761aa6f13d5b3f5014434d5c828e.jpg)