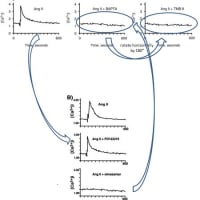
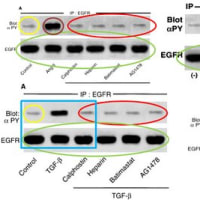
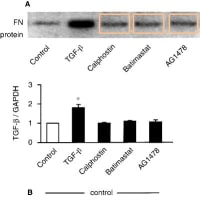
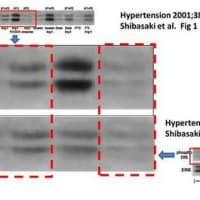
関西医大から京都府立医大への道 【2】からの続きです。
今回は次の二つの論文の読みくらべです。
一つ目は雑誌Biochemical and Biophysical Research Communicationsに掲載された論文(以下BBRC 論文とします)。
対抗するのは雑誌 Hypertensionに掲載のもの(以下Hypertension 論文)。
両論文とも2001年の掲載です。
BBRC 論文の筆頭著者は責任著者も兼ねています。
当時関西医大内科に属していたこの著者はHypertension 論文ではセカンドですが責任著者をつとめています。
BBRC These differences might be due to variation of cell types or reflect the complexity of the network involved in negative regulation of ERK activity. Thus, further dissection of the AT2 signaling pathway and identification of cross-point with Pyk2-JNK intracellular cascade, may provide new perspectives for pharmacological targeting of proliferative diseases.
Hypertension These differences might be due to a variation of cell types or might reflect the complexity of the network involved in negative regulation of ERK activity. Thus, further dissection of the AT2 signaling pathway and identification of the cross-point with EGFR cascades may provide new perspectives for pharmacological targeting of proliferative diseases and a unique example of negative cross-talk in growth signals.
BBRC However, the molecules interacting with SHP-1 were not defined in these earlier studies. Interestingly, Li et al. reported that platelet thrombin receptor causes SHP-1 tyrosine-phosphorylation in a PTX dependent manner, and suggested the role of tyrosine kinases linked to the thrombin receptor by Gi-protein.
Hypertension However, the molecules interacting with SHP-1 were not defined in these earlier studies. Li et al reported that platelet thrombin receptor causes SHP-1 tyrosine phosphorylation in a PTX-dependent manner and suggested the role of tyrosine kinases linked to the thrombin receptor by Gi protein.
BBRC Recently, a structural model for SHP-1 was proposed, in which SH2 domains of SHP-1 were shown to be capable of interacting with its C terminus in a phosphotyrosine-dependent manner and thereby drive the PTPase domain in an inactive conformation.
Hypertension Recently, a structural model for SHP-1 was proposed in which SH2 domains of SHP-1 were shown to be capable of interacting with its C terminus in a phosphotyrosine-dependent manner and thereby drive the PTPase domain in an inactive conformation.
BBRC It is possible that the conformational change of SHP-1 induced by AT2 causes an increased association of SHP-1 with downstream molecules of Pyk2, leading to JNK inactivation. Thus, we speculated that AT2 has the capacity to disrupt this intramolecular interaction.
Hypertension It is possible that the conformational change of SHP-1 induced by AT2 leads to the increased association of SHP-1 with EGFR and forms the basis for activation toward the receptor as observed in our study. Thus, AT2 may have the capacity to disrupt this intramolecular interaction.
BBRC SHP-1 is predominantly expressed in hematopoietic cells and plays a key role in hematopoiesis. Although the role of SHP-1 in VSMC has not been defined in detail, the present study suggested a novel function of SHP-1 in AT2-mediated JNK inactivation followed by a growth inhibitory action.
Hypertension SHP-1 is predominantly expressed in hematopoietic cells and plays a key role in hematopoiesis. Although the role of SHP-1 in VSMCs has not been defined in detail, the present study suggested a novel function of SHP-1 in AT2-mediated ERGFR inactivation followed by a growth inhibitory action.
BBRC Since SHP-1 was reported to interact with SHP-2, further studies are required to define the relationship between SHP-1 and SHP-2 in the mechanism for AT2 activation.
Hypertension Because SHP-1 was reported to interact with SHP-2, further studies are required to define the relationship between SHP-1 and SHP-2 in the mechanism of AT2 activation.
まただらだらと長くなりましたが、以上両論文の一部を読みくらべて、いかが思われましたか?
関西医大から京都府立医大への道 【4】に続きます。











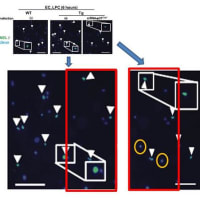
![画像類似の論文#16 (Kidney Int. 2011 Dec 7. doi: 10.1038/ki.2011.403. [Epub ahead of print])](https://blogimg.goo.ne.jp/image/upload/f_auto,q_auto,t_image_square_m/v1/user_image/21/de/685e525c47bce551c122291973de56dd.jpg)
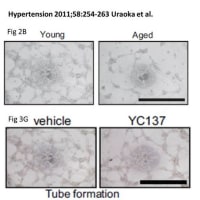
![画像類似の論文#16 (Kidney Int. 2011 Dec 7. doi: 10.1038/ki.2011.403. [Epub ahead of print])](https://blogimg.goo.ne.jp/image/upload/f_auto,q_auto,t_image_square_m/v1/user_image/57/ac/4bed761aa6f13d5b3f5014434d5c828e.jpg)