Dr Mark Trozzi(マーク・トロッツィ博士)がCOVID-19 mRNAワクチンによる障害報告に関する査読済み論文の約1,000件を掲載したサイトを作っています。
1000 peer reviewed articles on “Vaccine” injuries
その中で8番目に多くの件数が報告されている「アナフィラキシー(Anaphylaxis)」の論文リストを転載させていただきます。
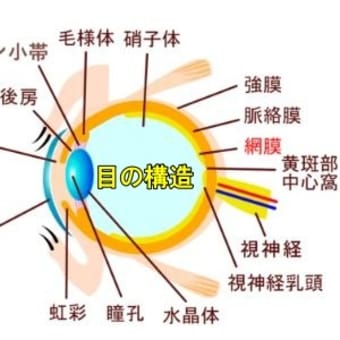
アナフィラキシー状態。生命を脅かす可能性のある重篤なアレルギー反応。国内では、アナフィラキシーを起こした人の9割以上は女性でした。使われている米ファイザー製と米モデルナ製のワクチンには、ポリエチレングリコール(PEG)が含まれています。化粧品の成分にもなる化合物です。これがアナフィラキシーの原因物質となっている可能性があります。
顔色不良、口周囲チアノーゼ、不活発(声を出さなくなる)。顔面チアノーゼ(軽微)が発現した実績があります。
掲載論文数:30
COVID-19 vaccine-associated anaphylaxis: a statement from the Anaphylaxis Committee of the World Allergy Organization:
https://www.sciencedirect.com/science/article/pii/S1939455121000119
.
Allergic reactions, including anaphylaxis, after receiving thefirst dose of the Pfizer-BioNTech COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/33475702/
Allergic reactions, including anaphylaxis, after receiving thefirst dose of Pfizer-BioNTech COVID-19 vaccine ? United States, December 14-23, 2020:
https://pubmed.ncbi.nlm.nih.gov/33444297/
Allergic reactions, including anaphylaxis, after receivingfirst dose of Modern COVID-19 vaccine ? United States, December 21, 2020-January 10, 2021:
https://pubmed.ncbi.nlm.nih.gov/33507892/
Reports of anaphylaxis after coronavirus disease vaccination 2019, South Korea, February 26-April 30, 2021:
https://pubmed.ncbi.nlm.nih.gov/34414880/
Reports of anaphylaxis after receiving COVID-19 mRNA vaccines in the U.S.-Dec 14, 2020-Jan 18, 2021: https://pubmed.ncbi.nlm.nih.gov/33576785/
Immunization practices and risk of anaphylaxis: a current, comprehensive update of COVID-19 vaccination data:
https://pubmed.ncbi.nlm.nih.gov/34269740/
Relationship between pre-existing allergies and anaphylactic reactions following administration of COVID-19 mRNA vaccine:
https://pubmed.ncbi.nlm.nih.gov/34215453/
Anaphylaxis Associated with COVID-19 mRNA Vaccines: Approach to Allergy Research:
https://pubmed.ncbi.nlm.nih.gov/33932618/
Allergic reactions and anaphylaxis to LNP-based COVID-19 vaccines:
https://pubmed.ncbi.nlm.nih.gov/33571463/
Cumulative adverse event report of anaphylaxis following injections of COVID-19 mRNA vaccine (Pfizer-BioNTech) in Japan: thefirst month report:
https://pubmed.ncbi.nlm.nih.gov/34347278/
COVID-19 vaccines increase the risk of anaphylaxis:
https://pubmed.ncbi.nlm.nih.gov/33685103/
Biphasic anaphylaxis after exposure to thefirst dose of the Pfizer-BioNTech COVID-19 mRNA vaccine COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34050949/
Polyethylene glycol (PEG) is a cause of anaphylaxis to Pfizer / BioNTech mRNA COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/33825239/
Elevated rates of anaphylaxis after vaccination with Pfizer BNT162b2 mRNA vaccine against COVID-19 in Japanese healthcare workers; a secondary analysis of initial post-approval safety data:
https://pubmed.ncbi.nlm.nih.gov/34128049/
.IgE-mediated allergy to polyethylene glycol (PEG) as a cause of anaphylaxis to COVID-19 mRNA vaccines:
https://pubmed.ncbi.nlm.nih.gov/34318537/
Anaphylactic reactions to COVID-19 mRNA vaccines: a call for further studies:
https://pubmed.ncbi.nlm.nih.gov/33846043/ 188
.
Anaphylaxis following Covid-19 vaccine in a patient with cholinergic urticaria:
https://pubmed.ncbi.nlm.nih.gov/33851711/
Anaphylaxis induced by CoronaVac COVID-19 vaccine: clinical features and results of revaccination:
https://pubmed.ncbi.nlm.nih.gov/34675550/
Anaphylaxis after Modern COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34734159/
Sex differences in the incidence of anaphylaxis to LNP-mRNA vaccines COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34020815/
Allergic reactions, including anaphylaxis, after receiving thefirst dose of Pfizer-BioNTech COVID-19 vaccine ? United States, December 14 to 23, 2020:
https://pubmed.ncbi.nlm.nih.gov/33641264/
Allergic reactions, including anaphylaxis, after receiving thefirst dose of Modern COVID-19 vaccine ? United States, December 21, 2020 to January 10, 2021:
https://pubmed.ncbi.nlm.nih.gov/33641268/
Prolonged anaphylaxis to Pfizer 2019 coronavirus disease vaccine: a case report and mechanism of action:
https://pubmed.ncbi.nlm.nih.gov/33834172/
Anaphylaxis reactions to P? zer BNT162b2 vaccine: report of 3 cases of anaphylaxis following vaccination with Pfizer BNT162b2:
https://pubmed.ncbi.nlm.nih.gov/34579211/
Biphasic anaphylaxis afterfirst dose of 2019 messenger RNA coronavirus disease vaccine with positive polysorbate 80 skin test result:
https://pubmed.ncbi.nlm.nih.gov/34343674/
Biphasic anaphylaxis after exposure to thefirst dose of Pfizer-BioNTech COVID-19 mRNA vaccine COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34050949/
Iguchi, T., Umeda, H., Kojima, M., Kanno, Y., Tanaka, Y., Kinoshita, N., & Sato, D. (2021). Cumulative Adverse Event Reporting of Anaphylaxis After mRNA COVID-19 Vaccine (Pfizer-BioNTech) Injections in Japan: The First-Month Report. Drug Saf, 44(11), 1209-1214. doi:10.1007/s40264-021-01104-9
https://www.ncbi.nlm.nih.gov/pubmed/34347278
Team, C. C.-R., Food, & Drug, A. (2021). Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine ? United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep, 70(2), 46-51. doi:10.15585/mmwr.mm7002e1.
https://www.ncbi.nlm.nih.gov/pubmed/33444297
さらにひどい症状だとアナフィラキシーショックがあり、複数の臓器に症状がみられます。
〈1〉皮膚、粘膜症状(全身の発疹やかゆみ、口や舌のただれ)
〈2〉呼吸器症状(呼吸困難、強いせきなど)
〈3〉循環器症状(血圧低下、意識障害)
〈4〉消化器症状( 嘔吐おうと 、下痢など)