Primordial GATA6 macrophages function as extravascular platelets in sterile injury
GATA6+ macrophages resident in body cavities exhibit both phagocytic and repair functions. However, the mechanisms by which these cells can identify and migrate to sites of injury have remained unclear. Using intravital imaging of mouse peritoneal cavities, Zindel et al. report that GATA6+ macrophages rapidly assemble clot-like structures in a process strongly analogous to thrombosis (see the Perspective by Herrick and Allen). The formation of these aggregates requires the expression of macrophage scavenger receptor domains and acts to plug wounds and promote healing. This pathway can be inadvertently activated during medical procedures, when macrophage aggregates can promote the generation of abdominal scar tissue known as adhesions. Inhibition of macrophage scavenger receptors may therefore be a useful therapeutic approach after surgeries that cause injury to body cavities. Science , this issue p. [eabe0595][1]; see also p. [993][2] ### INTRODUCTION Most multicellular organisms have a major body cavity that harbors immune cells. In primordial species such as the purple sea urchin, these cells—called coelomocytes—fulfill dual functions. Sea urchin coelomocytes clear pathogens from the peritoneal compartment, but they have also been shown to form multicellular aggregates that adhere to injured tissue and are crucial for repair. In mammals, the peritoneal, pleural, and pericardial cavities are filled with vast numbers of resident GATA6+ cavity macrophages. The role of peritoneal cavity macrophages as phagocytes in clearing pathogens has been established for decades. Recent evidence suggests that these cells migrate to injuries within the peritoneal cavity, where they have been shown to promote tissue repair. ### RATIONALE It remains unclear how cavity macrophages, which are suspended in the fluid phase (peritoneal fluid), can identify injuries, which can be several thousand micrometers away, and how they can exhibit chemotaxis over that distance through a fluid-filled compartment that is under constant convective flow. In this study, we developed an intravital microscopy (IVM) model to study the dynamics and molecular mechanisms of resident GATA6+ macrophage recruitment in the peritoneal cavity after injury. ### RESULTS By using inverted multiphoton IVM with extremely sensitive non-descanned hybrid detectors, we were able to image the peritoneal cavity through the intact abdominal wall in living animals. The tracks of the rapidly moving peritoneal macrophages showed that they passively traversed the peritoneal cavity in a respiration-dependent and seemingly random pattern. Next, we used a focused high-power infrared laser beam to induce focal injuries to the peritoneum, and we imaged the subsequent immune response. We found that peritoneal macrophages were rapidly recruited through a two-step process: (i) an initial tether of macrophages to the injury site, followed by (ii) secondary tethers that formed an aggregate reminiscent of a thrombus-like structure in response to injury. Macrophage aggregation mirrored and rivaled the speed of platelet aggregation (thrombus formation) in the adjacent vasculature. By probing the transcriptome of peritoneal macrophages and a targeted series of knockout and inhibition IVM experiments, we found that peritoneal macrophage aggregation was independent of canonical mammalian adhesion molecules such as integrins, selectins, and immunoglobulin-like adhesion molecules. Instead, peritoneal macrophage aggregation was dependent on primordial scavenger receptor cysteine-rich (SRCR) domains. SRCR domains are highly conserved among species, with many homologs expressed by sea urchin coelomocytes and sea sponges, and some of these proteins have been identified as cell-cell adhesion molecules in these primordial organisms. Aggregates of cavity macrophages physically sealed injuries and promoted rapid repair of focal peritoneal lesions. However, in abdominal surgery models that reflect iatrogenic surgical situations in which the peritoneal cavity is opened and foreign suture material is introduced, these cavity macrophages formed extensive aggregates that promoted the growth of intra-abdominal scar tissue called peritoneal adhesions. These peritoneal adhesions cause substantial morbidity for patients and considerable costs for health care systems. We showed that the number and tenacity of peritoneal adhesions was significantly reduced by either depleting peritoneal macrophages or therapeutically inhibiting their scavenger receptor–dependent recruitment and aggregation. ### CONCLUSION Our results unveil a platelet-like extravascular fluid-phase response by macrophages. This rapid response seals the peritoneal leaks within minutes and serves an important function in repairing small injuries such as focal thermal or laser-induced peritoneal injuries. We hypothesize that such focal injuries reflect a type of injury for which the immune system has evolved a beneficial response. By contrast, iatrogenic procedures, such as abdominal surgery involving implantation of foreign material, reflect a type of injury that has no evolutionary precedent. In this scenario, peritoneal macrophages may cause detrimental scarring, instead of restitution ad integrum, in an attempt to repair the wound. Thus, macrophage aggregation and its inhibition by scavenger receptor antagonists are of clinical importance and may provide a therapeutic target to prevent scar formation after surgery in the peritoneal cavity. Furthermore, these findings may extend to other cavities, including pleural and pericardial spaces. ![Figure][3] Aggregation of GATA6+ peritoneal cavity macrophages in response to laser-induced peritoneal injury. ( A ) Human, mouse, and sea urchin coelomic cavities (mouse example enlarged) with circulating coelomocytes and macrophages. Injuries were induced by a multiphoton laser. ( B ) IVM image immediately after injury. Scale bar, 50 μm. ( C ) IVM image 30 min after injury. Scale bar, 50 μm. ( D ) Aggregation was dependent on scavenger receptors and a (yet unknown) polyanionic ligand. Most multicellular organisms have a major body cavity that harbors immune cells. In primordial species such as purple sea urchins, these cells perform phagocytic functions but are also crucial in repairing injuries. In mammals, the peritoneal cavity contains large numbers of resident GATA6+ macrophages, which may function similarly. However, it is unclear how cavity macrophages suspended in the fluid phase (peritoneal fluid) identify and migrate toward injuries. In this study, we used intravital microscopy to show that cavity macrophages in fluid rapidly form thrombus-like structures in response to injury by means of primordial scavenger receptor cysteine-rich domains. Aggregates of cavity macrophages physically sealed injuries and promoted rapid repair of focal lesions. In iatrogenic surgical situations, these cavity macrophages formed extensive aggregates that promoted the growth of intra-abdominal scar tissue known as peritoneal adhesions. [1]: /lookup/doi/10.1126/science.abe0595 [2]: /lookup/doi/10.1126/science.abg5416 [3]: pending:yes













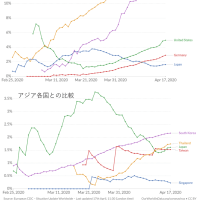
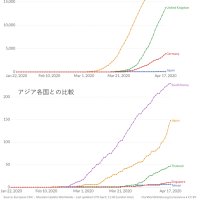
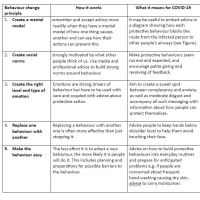
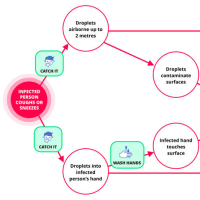
※コメント投稿者のブログIDはブログ作成者のみに通知されます