Tetrahedrons
Only God knows where I am heading.
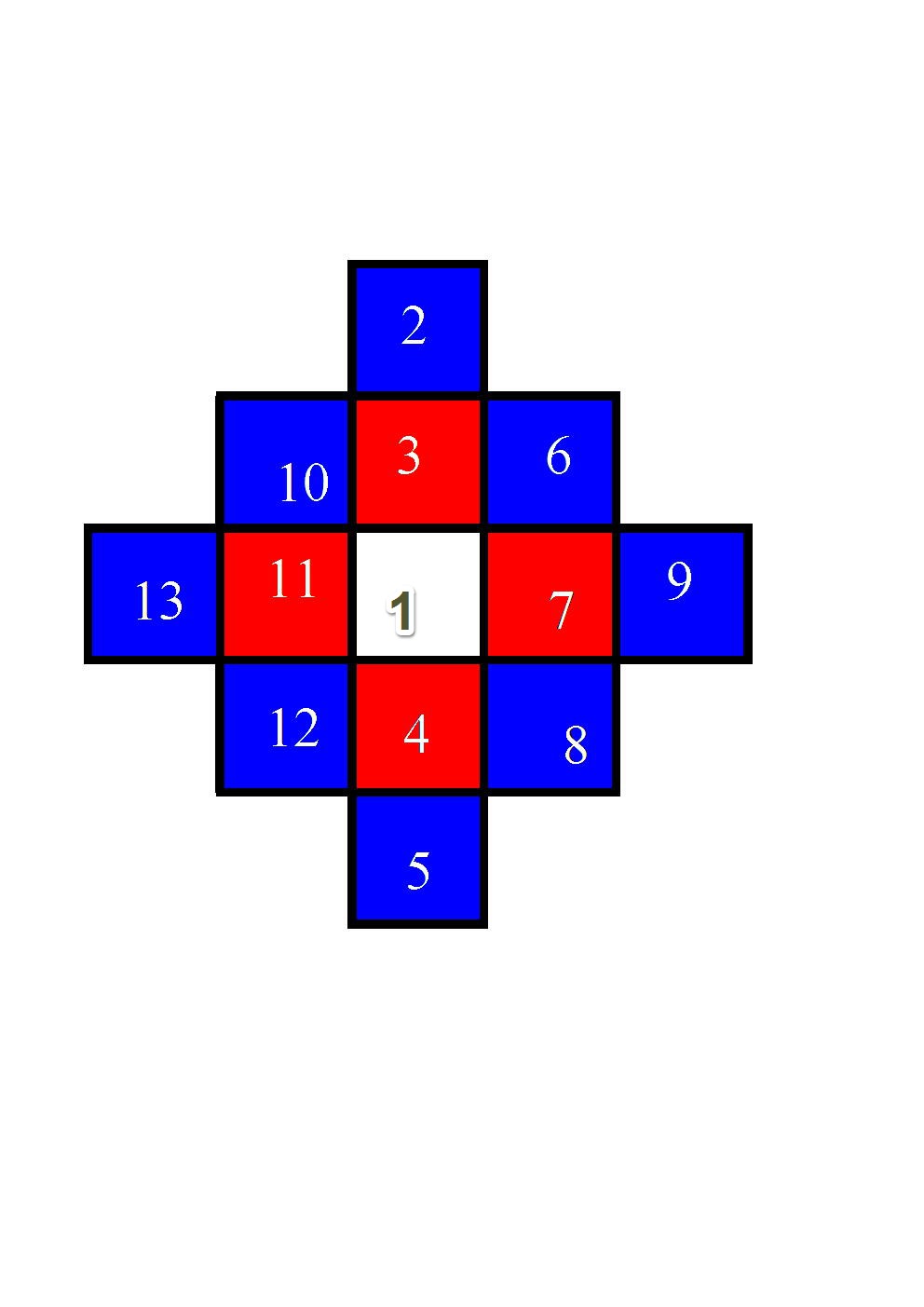
Above schematic is my attempt to try and understand how cubes might be connected to form a certain structure. According to my Web survey a lot of metals are formed from cube structured seed crystal. Common salt is also another example.
One thing we must perhaps remember is that crystal formation in condensed environment such as free waters is different from crystal formation in sparse environment such as turbulent air blocks.
In turbulent air in the atmosphere water molecules are expected to be doing all sorts of free motion, translational, rotational and vibrational, or mixture of all these, I think.
Ice formation in free waters will be different. Anyway, if you take a look at above schematic you will begin to see that it is going to form into a sphere, eventually.
The growth starts with the seed crystalet, coded as #1 in white. The first layer of attached crstalets (3,4,7, and 11) is indicated in red, and the second in blue and so on.
With this crystal growth modelling we can see that there is no preferential growth direction. Crystal is growing in all directions and evenly through statistical process of accidental molecular attachments to existing and inviting(?) faces, all six of them, initially at least, and less faces for attachment per cube once growth is started.
All above is just using the schematic representation. Now, let us take a look at physical modelling processes.
First, we look at the following five pictures. The most important thing here to realize is that only the same two crystalet pieces are used. Each face is numbered for clarity.

Here above, you start out from two pieces bang on.
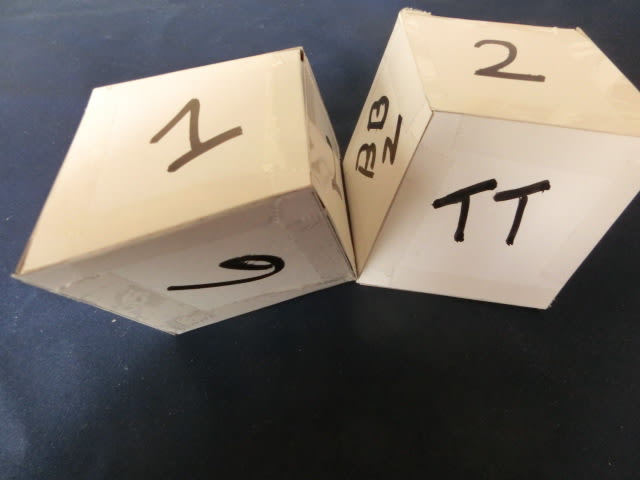
As you rotate the right hand piece only you begin to see that the bonding gets weaker, because they are no longer sharing the same corners where bonding atoms are located.

Weak bonding continues.

Actually, they are almost breaking apart!

OK, here, we are back to where we started out from. However, bonding faces are now different. The whole combined structure remains the same.
We now look at four more pictures as per below. The left hand piece remains the same. However, the right hand piece is now tilted in different orientation and rotated as before.

This above is just one rotation from the last picture above. The structure is already falling apart.

This process continues.
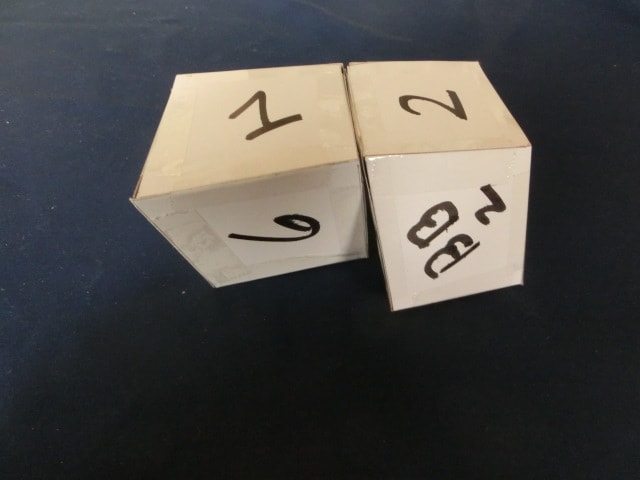
Same here.

Here above, we are again back to where we started from. Same faces are bang on.
In all above, I just wanted to show you that with the same two pieces (exactly the same two pieces) only a specific structure is possible and always with the same faces bonded together.
In a way, this is promising, because you have a certain rule here, and that rule is expected to apply in all stages of crystal formation, leading eventually and hopefully to the birth of star like crystal...
However, with the following pictures you will begin to see that the whole thing is not that easy.

In above photo, I am trying to connect three crystalets in a straight line. There is a small mismatch between the left two members, but this is due to the inaccuracy in constructing the left most model.

As you can see above, three crystalets are pefectly aligned in a straight line. So, in theory and practice this connecting process can continue to form a lone line of molecules.
Now, take a look at the next photo shown below. Here, I am trying to see if other molecules with perhaps different orientation might attatch to the existing line of molecules on the left.


The answer is affirmative, as you see above. However, all this presents a (to me, at least) insurmountable problem.
When I produced these paper models they were made by the same template. When I formed them into the structure we are seeing I did not doubt for a moment that I am making identical pieces.

However, above photo pushed me off the cliff. There are two different types of seemingly identical molecules. It was not intentional on my part. They were made quite accidentally. I am dead certain.
Above two molecules can connect, as I expected. And, below two can connect as well, but differently! They only connect at an angle, not in a straight line like molecules above in this same photo.
The whole edifice of my simulatory attempt collapsed at this very moment. It took me a long while before I came to. I knew instinctively that the matter is getting out of my hands.
So, I resorted to the web knowledge again and discovered, unlike my imagination, that ice crystals are tetrahadrons in shape! Tha is to say, they have only 4 faces, unlike my 6 faced crystals!
I knew then immediately that the whole thing is now out of control. So, out of curiosity I explored still further and discovered a few other things. I will talk about them soon...
I carry below 4 picrures, just to show the whole ting is a little more complicated that I initially imagined. They all come from the same author who published some papers in the same area, as follows.

This group naturally have access to a super computer and their molecular dynamics simulation looks fantastic.
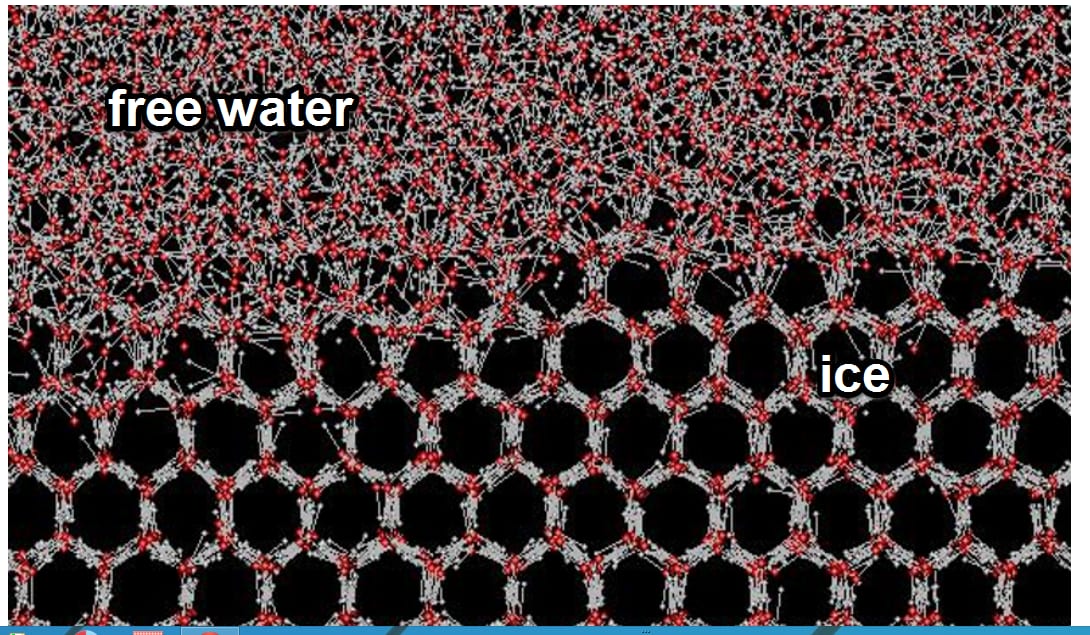
This above is showing us the interface between free water molecules and ice by molecular dynamical simulation. Situation is somewhat different from ice formation in turbulent air, but the idea is there.
Next photo is more convincing(?). It is about preferential growth of arms.
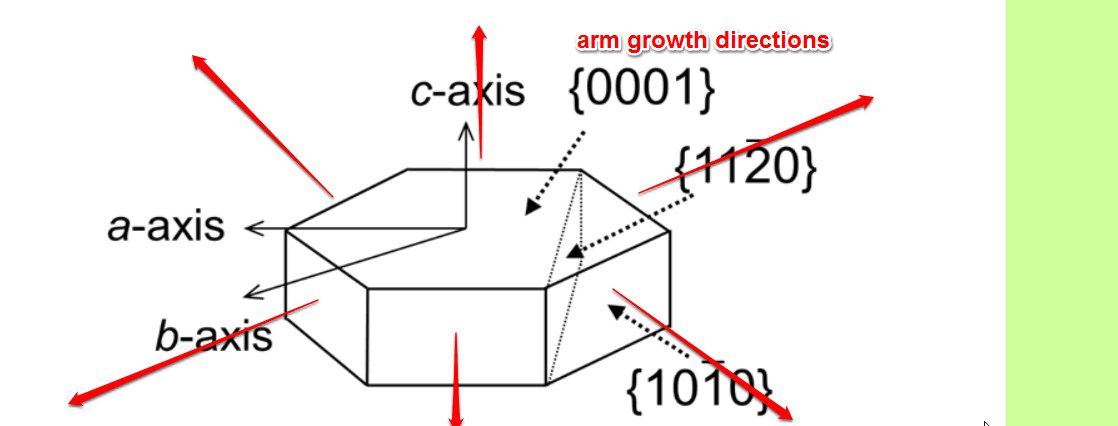
There must be a lot of chemistry or physics involved, from the initial tetrahedronic structure to this macroscopic crystal formation. I salute to those fellows who share my naive interest in the formation of ice crystals.
Case closed.




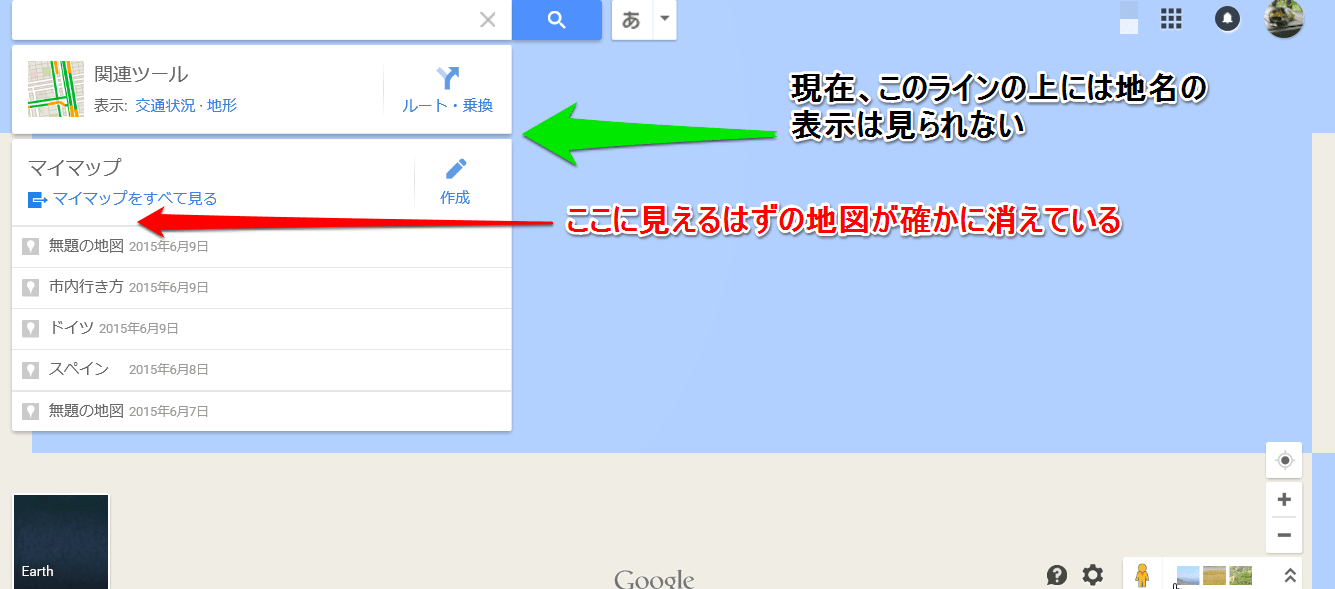
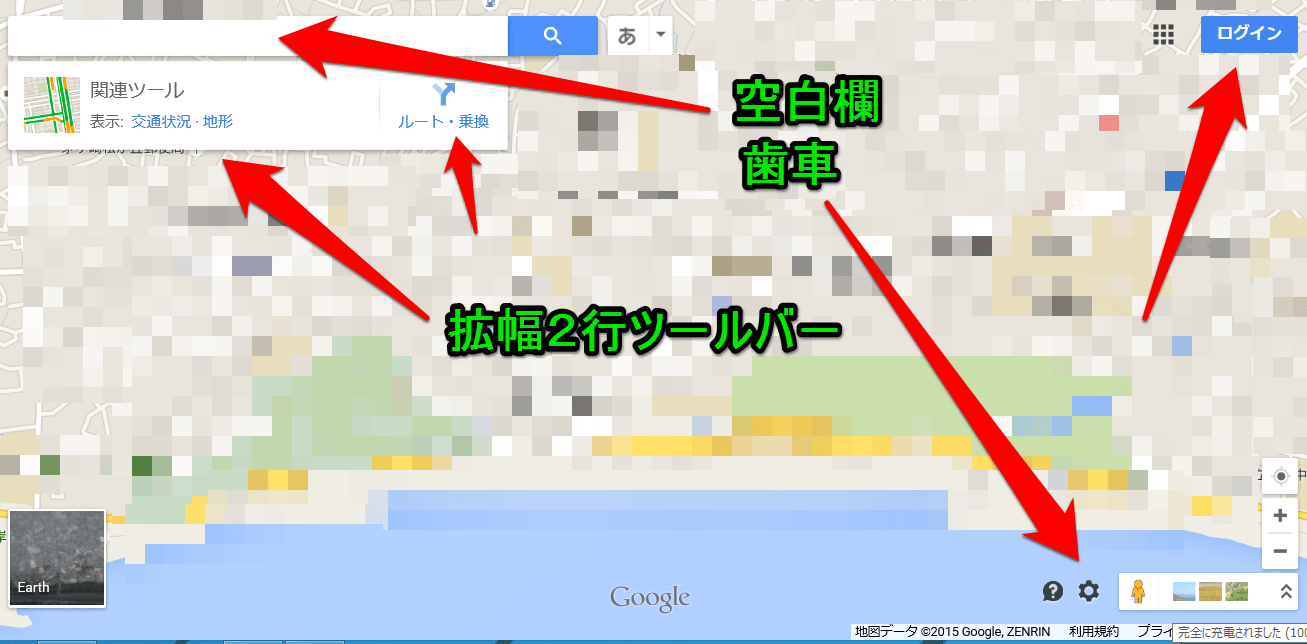
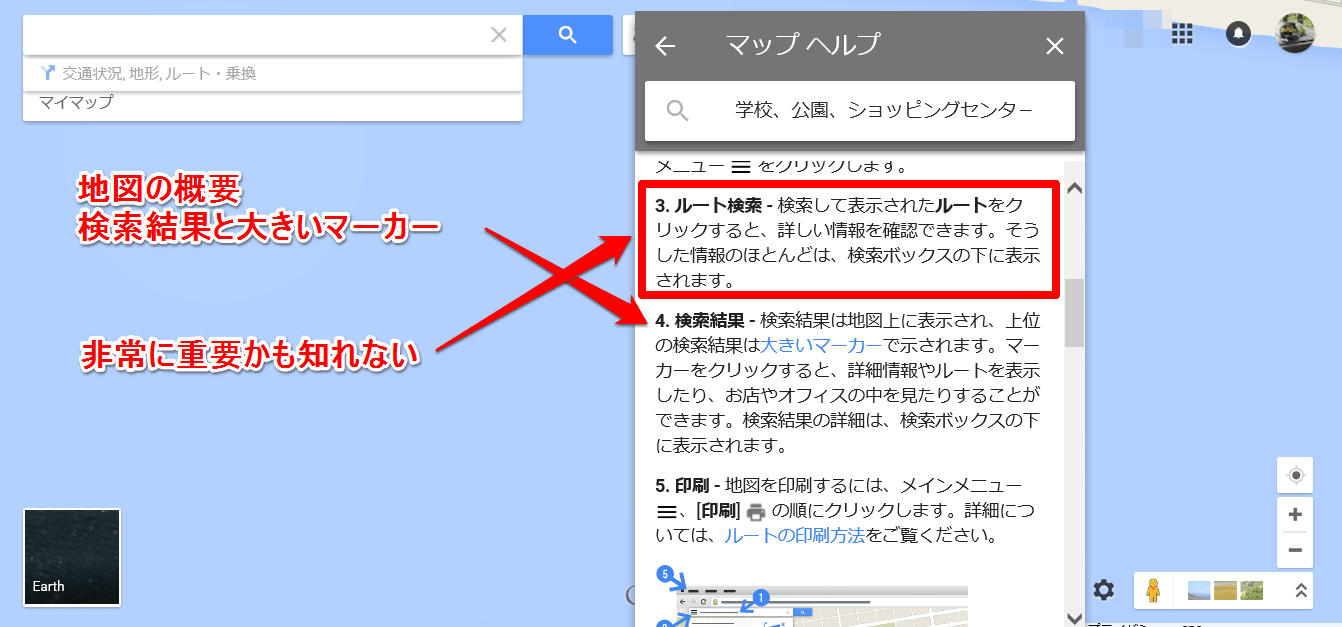 これは地図の概要の一部だが、ここで初めて「マ-カ-」と言う文字が見え始めた。項目の3が言っているのは、恐らく検索結果の青いラインをクリックすると、何か重要な情報が出て来るとの事だろう。試してみなければ、それが何かは判らないが。
これは地図の概要の一部だが、ここで初めて「マ-カ-」と言う文字が見え始めた。項目の3が言っているのは、恐らく検索結果の青いラインをクリックすると、何か重要な情報が出て来るとの事だろう。試してみなければ、それが何かは判らないが。