US9190698 (Lithium-ion electrolytes with improved safety tolerance to high voltage systems)
"The invention discloses various embodiments of electrolytes for use in(ための、用)lithium-ion batteries, the electrolytes having improved safety and the ability to operate with high capacity anodes and high voltage cathodes. In one embodiment there is provided(倒置)an electrolyte for use in a lithium-ion battery comprising(から成る)an anode and a high voltage cathode. The electrolyte has a mixture of a cyclic carbonate of ethylene carbonate (EC) or mono-fluoroethylene carbonate (FEC) co-solvent, ethyl methyl carbonate (EMC), a flame retardant additive, a lithium salt, and an electrolyte additive that improves compatibility and performance of the lithium-ion battery with a high voltage cathode. The lithium-ion battery is charged to a voltage in a range of from about 2.0 V (Volts) to(範囲)about 5.0 V (Volts)." (Abstract)
"Lithium-ion (“Li-ion”) cells typically include a carbon (e.g., coke or graphite) anode intercalated with(挿入)lithium ions to form LixC; an electrolyte consisting of a lithium salt dissolved in one or more(一種類以上の)organic solvents; and a cathode made of(から成る、作製、作られた、構成)an electrochemically active material, typically an insertion compound, such as LiCoO2. During cell discharge, lithium ions pass from the carbon anode, through the electrolyte to the cathode, where the ions are taken up(引き出す?)with the simultaneous release of electrical energy. During cell recharge, lithium ions are transferred back to the anode, where they reintercalate into(再挿入)the carbon matrix."
"Future NASA missions aimed at exploring Mars, the Moon, and the outer planets require rechargeable batteries that can operate effectively over a wide temperature range (−60° C. (Celsius) to +60° C. (Celsius)) to satisfy the requirements of various applications, including: Landers (lander spacecraft), Rovers (surface rover spacecraft), and Penetraters (surface penetrator spacecraft). Some future applications typically(典型)will require high specific energy batteries that can operate at very low temperatures, while still providing adequate(十分な)performance and stability at higher temperatures. In addition, many of these applications envisioned(想定、計画、予測)by the ESRT (Exploration Systems Research and Technology) program will require improved safety, due to their use by humans. Lithium-ion rechargeable batteries(複数形;一般)have the demonstrated characteristics of high energy density, high voltage, and excellent cycle life. Currently, the state-of-the-art lithium-ion system(定冠詞、断定的;不定冠詞だと変に具体的になって聞き手、読み手の好奇心を下手にくすぐるのではないか?)has been demonstrated to operate over a wide range of temperatures (−40° C. to +40° C.), however, abuse(過酷な)conditions such as being exposed to high temperature, overcharge, and external shorting, can often lead to cell rupture and fire. The nature of the electrolyte can greatly affect the propensity of the cell/battery to(傾向、易さ)catch fire, given the flammability of the organic solvents used within. Therefore, extensive effort has been devoted recently to developing(近年、開発のため鋭意努力)non-flammable electrolytes to reduce the flammability of the cell/battery."
"Desired properties for Li-ion electrolytes can include high conductivity over a wide temperature range (e.g., 1 mS (milli-Siemens) cm−1 from −60° C. to +60° C.); good electrochemical stability over a wide voltage range (e.g., 0 to 4.5V (volts)) with minimal oxidative degradation of solvents/salts; good chemical stability; good compatibility with a chosen electrode couple, including good SEI (solid electrolyte interface) characteristics on the electrode and facile lithium intercalation/de-intercalation kinetics; good thermal stability; good low temperature performance throughout the life of the cell, including good resilience to high temperature exposure and minimal impedance build-up with cycling and/or storage; and low toxicity. Since the flammability of the electrolyte solution in Li-ion batteries is a major concern, significant research has been devoted to developing electrolyte formulations with increased safety. Known electrolytes used in state-of-the-art Li-ion cells have typically comprised binary mixtures of organic solvents, for example, high proportions of ethylene carbonate, propylene carbonate or dimethyl carbonate, within which is dispersed a lithium salt, such as lithium hexafluorophosphate (LiPF6). Examples may include 1.0 M (molar) LiPF6 in a 50:50 mixture of ethylene carbonate/dimethyl carbonate, or ethylene carbonate/diethyl carbonate. More recently, electrolytes have also been developed which combine more than two solvents and/or have incorporated the use of electrolyte additives to address specific performance goals."
Lithium-ion battery (Wikipedia)
"The three primary functional components of a lithium-ion battery are the positive and negative electrodes and electrolyte. Generally, the negative electrode of a conventional lithium-ion cell is made from(から成る、作られる)carbon. The positive electrode is(である、から成る)a metal oxide, and the electrolyte is a lithium salt in an organic solvent.[45] The electrochemical roles of the electrodes reverse(逆になる)between anode and cathode, depending on the direction of current flow through the cell.
The most commercially popular negative electrode is graphite. The positive electrode is generally one of three materials: a layered oxide (such as lithium cobalt oxide), a polyanion (such as lithium iron phosphate) or a spinel (such as lithium manganese oxide).[46]
The electrolyte is typically a mixture of organic carbonates such as ethylene carbonate or diethyl carbonate containing complexes of lithium ions.[47] These non-aqueous electrolytes generally use non-coordinating(非配位性)anion salts such as lithium hexafluorophosphate (LiPF
6), lithium hexafluoroarsenate monohydrate (LiAsF
6), lithium perchlorate (LiClO
4), lithium tetrafluoroborate (LiBF
4) and lithium triflate (LiCF
3SO
3)."
"The participants(関与する)in the electrochemical reactions in a lithium-ion battery are the negative and positive electrodes with the electrolyte providing a conductive medium for Lithium-ions to move between the electrodes.
Both electrodes allow lithium ions to move in and out of their interiors. During insertion (or intercalation)(挿入)ions move into the electrode. During the reverse process, extraction (or deintercalation)(脱離), ions move back out. When a lithium-ion based cell is discharging(放電している), the positive Lithium ion moves from the negative electrode (usually graphite = " " below) and enters the positive electrode (lithium containing compound). When the cell is charging(充電している; cf. being charged), the reverse occurs.
" below) and enters the positive electrode (lithium containing compound). When the cell is charging(充電している; cf. being charged), the reverse occurs.
Useful work is performed when electrons flow through a closed external circuit. The following equations show one example of the chemistry, in units(単位)of moles, making it possible to use coefficient 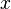 .
.
The cathode (marked +) half-reaction is:[54]

The anode (marked -) half reaction is:

The overall reaction has its limits. Overdischarge(過放電)supersaturates lithium cobalt oxide, leading to the production(生成、発生、生じる) of lithium oxide,[55] possibly by the following irreversible reaction:

Overcharge(過充電)up to 5.2 volts leads to the synthesis of cobalt(IV) oxide, as evidenced by x-ray diffraction:[56]
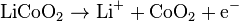
In a lithium-ion battery the lithium ions are transported to and from the positive or negative electrodes by oxidizing the transition metal, cobalt (Co)(遷移金属、すなわちコバルト), in Li
1-xCoO
2 from Co3+
to Co4+
during charge, and reduced from Co4+
to Co3+
during discharge. The cobalt electrode reaction is only reversible for x < 0.5, limiting the depth of discharge allowable. This chemistry was used in the Li-ion cells developed by Sony in 1990."
"Electrolytes[edit]
The cell voltages given in the Electrochemistry section are larger than the potential at which aqueous solutions will electrolyze(電解する).
Liquid electrolytes(液体電解質)in lithium-ion batteries consist of(から成る)lithium salts, such as LiPF
6, LiBF
4 or LiClO
4 in an organic solvent, such as ethylene carbonate, dimethyl carbonate, and diethyl carbonate.[57] A liquid electrolyte acts as(作用、機能する)a conductive pathway for the movement of cations passing from the positive to the negative electrodes during discharge(放電中). Typical conductivities(複数形)of liquid electrolyte at room temperature (20 °C (68 °F)) are in the range of 10 mS/cm, increasing by approximately 30–40% at 40 °C (104 °F) and decreasing slightly at 0 °C (32 °F).[58]
The combination of linear and cyclic carbonates (e.g., ethylene carbonate (EC) and dimethyl carbonate (DMC)) offers(により得ることができる)high conductivity and SEI-forming ability. A mixture of a high ionic conductivity and low viscosity carbonate solvents is needed, because the two properties are mutually exclusive in a single material.[59]
Organic solvents easily decompose(分解する)on the negative electrodes during charge(充電中). When appropriate organic solvents are used as the electrolyte, the solvent decomposes on initial charging and forms a solid layer called the solid electrolyte interphase (SEI),[60] which is electrically insulating yet provides significant ionic conductivity. The interphase prevents further decomposition of the electrolyte after the second charge. For example, ethylene carbonate is decomposed at a relatively high voltage, 0.7 V vs. lithium, and forms a dense and stable interface.[61]
Composite electrolytes based on POE (poly(oxyethylene)) provide(が得られる)a relatively stable interface.[62][63] It can be either solid (high molecular weight) and be applied in dry Li-polymer cells, or liquid (low molecular weight) and be applied in regular Li-ion cells.
Room temperature ionic liquids (RTILs) are another approach to(するための方法、手段)limiting the flammability and volatility of organic electrolytes.[64]"
"Materials[edit]
The increasing demand for batteries has led vendors and academics to focus on improving the energy density, operating temperature, safety, durability, charging time, output power, and cost of lithium ion battery solutions. The following materials have been used in commercially available cells. Research into other materials continues.
Cathode materials are generally constructed out of(から成る、構成される)two general materials: LiCoO¬2 and LiMn2O4. The cobalt-based material develops a pseudo tetrahedral structure that allows for two-dimensional Lithium ion diffusion.[78] The cobalt-based cathodes are ideal due to their high theoretical specific heat capacity, high volumetric capacity, low self-discharge, high discharge voltage, and good(良好)cycling performance. Limitations include the high cost of the material, slight toxicity, and low thermal stability.[79] The manganese-based materials adopt a cubic crystal lattice system, which allows for three-dimensional Lithium ion diffusion.[78] Manganese cathodes are attractive(興味深い、注目されている)because manganese is cheaper and less toxic than other materials used. Limitations include the tendency for manganese to dissolve into the electrolyte during cycling leading to poor cycling stability for the cathode.[79] Cobalt-based cathodes are the most common however other materials are beginning to be developed to make cheaper and less toxic cathodes.[80]"
US7488465 (Solid state synthesis of lithium ion battery cathode material)
"Single-phase(単相)lithium-transition metal oxide compounds containing(含有)cobalt, manganese and nickel can be prepared by wet milling(湿式粉砕)cobalt-, manganese-, nickel- and lithium-containing oxides(含有酸化物)or oxide precursors to form(~して形成)a finely-divided(微粉化)slurry containing well-distributed cobalt, manganese, nickel and lithium, and heating the slurry to provide a lithium-transition metal oxide compound containing cobalt, manganese and nickel and having(有する)a substantially single-phase O3 crystal structure. Wet milling provides significantly shorter milling times(粉砕時間が短い)than dry milling and appears to(ようである)promote formation of single-phase lithium-transition metal oxide compounds. The time savings in the wet milling step more than offsets(補って余りある)the time that may be required to dry the slurry during the heating step." (Abstract)
"Lithium-ion batteries typically(通常、典型、一般的に)include an anode, and electrolyte and a cathode that contains lithium in the form of(の形で)a lithium-transition metal oxide. Transition metal oxides that have been used include cobalt dioxide(無冠詞), nickel dioxide and manganese dioxide."
"Lithium-transition metal oxide compounds in which(~している化合物)cobalt, manganese and nickel are each present in the crystal lattice can be referred to as(呼ぶ)four metal or quaternary cathode(4種の金属を有する、すなわち4元カソード?)compounds. Single-phase lattices containing appropriate amounts of these metals can provide(用いることにより得ることができる)especially desirable lithium-ion battery cathodes. For example, the quaternary compounds:
LiNi0.1Mn0.1Co0.8O2 (I)
Li(Co(1/3)Mn(1/3)Ni(1/3))O2 (II) and
Li(Li0.08Co0.15Mn0.375Ni0.375)O2 (III)
are of interest(対象となる、考えられる)if successfully formed as a single-phase (if multiple phases are present, then battery performance suffers(低下、悪化)). The equimolar manganese and nickel content in these three compounds is especially desirable and is believed to(考えられる)contribute to formation of a more stable crystal lattice."
"Unfortunately(残念ながら), it can be difficult to form a single-phase quaternary compound containing the transition metals cobalt, manganese and nickel in a lithium-containing crystal lattice. Attainment of a single-phase can be made easier by(容易に達成)excluding one or more of the transition metals manganese or nickel(選択肢のor)(e.g., to make a three metal or ternary system such as LiNi0.8Co0.2O2 or a two metal or binary system such as LiCoO2), but this may also decrease battery performance or introduce other problems. Attainment of a single-phase quaternary compound may be achieved by coprecipitation of mixed metal hydroxides as recommended and employed in U.S. Patent Application No. 2003/0022063A1 (Paulsen et al.) entitled “Lithiated Oxide Materials and Methods of Manufacture” by coprecipitation of mixed metal nitrates and metal hydroxides and as employed in Examples 19 and 20 of U.S. Patent Application No. 2003/0027048 A1 (Lu et al.) entitled “Cathode Compositions for Lithium-Ion Batteries”. However, coprecipitation requires filtration, repeated washing and drying and thus exhibits relatively limited throughput and high manufacturing costs(製造コストが高い)."
"Paulsen et al. also describes and employs in its Example 6 a high-energy ball milling and sintering(焼結)process to make certain lithium-transition metal oxide compounds having the formula:
Li(LixCoy(MnzNi1-z)1-x-y)O2 (IV)
where 0.4≦z≦0.65, 0<x≦0.16 and 0.1≦y≦0.3. U.S. Pat. No. 6,333,128 B1 (Sunagawa et al.) entitled “Lithium Secondary Battery” employs in its Examples A1 through A9 a powder mixing, baking and jet milling process to make certain lithium-transition metal oxide compounds having the formula:
LiaCobMncNi1-b-cO2 (V)
where 0≦a≦1.2, 0.01≦b≦0.4, 0.01≦c≦0.4 and 0.02≦b+c≦0.5. These Paulsen et al. and Sunagawa et al. processes involve solid state reactions and potentially offer higher throughput and lower manufacturing costs(製造コストの低減を可能とする)than processes based on coprecipitation. However, when we attempted to replicate some of the Paulsen et al. and Sunagawa et al. compounds using the described processes we obtained multiple phase(多相)compounds rather than the desired single-phase structure. Also, when we attempted to prepare the above-mentioned compounds of formulas I through III (which fall outside formulas IV and V) using a solid state reaction, we obtained(主語we)multiple phase compounds rather than the desired single-phase structure. By using about 15 wt. % excess lithium, we were able to(できた; cf. could)make compounds in the solid solution between LiCoO2 and Li2MnO3 by solid state reaction. The excess lithium aided(促進、支援)formation of a single-phase material, but the resulting product had poor electrochemical performance."
"Suitable(好適)cobalt-, manganese- and nickel-containing oxides or oxide precursors include cobalt hydroxide(単数形)(Co(OH)2), cobalt oxides(複数形)(e.g., Co3O4 and CoO), manganese carbonate (Mn2CO3), manganese oxide (MnO), manganese tetroxide (Mn3O4), manganese hydroxide (Mn(OH)2), basic manganese carbonate (Mn2CO3*xMn(OH)2), nickel carbonate (Ni2CO3), nickel hydroxide (Ni(OH)2), and basic nickel carbonate (Ni2CO3*xNi(OH)2), Preferably at least one of the manganese or(選択肢のor)nickel precursors is a carbonate."
"Suitable lithium-containing oxides and oxide precursors include lithium carbonate (Li2CO3) and lithium hydroxide (LiOH). If desired(必要に応じて), hydrates of the precursors can be employed.
The amounts of each oxide or oxide precursor typically are selected based on the composition of a targeted final compound(目的). A wide variety of targeted final compounds can be prepared. The plots shown in FIG. 1and FIG. 2 can assist in selecting a target. FIG. 1 is a triangular pyramidal plot whose vertices(頂点)A, B, C and D respectively(それぞれ)represent the compositions LiCoO2, LiMnO2, LiNiO2 and Li(Li1/3Mn2/3)O2. Vertices A, B and C thus(したがって) respectively represent maximum cobalt, manganese and nickel contents for binary lithium-transition metal oxide compounds containing these transition metals in the indicated stoichiometry. Point E located midway along(途中)edge BC represents the composition LiMn1/2Ni1/2O2. Points within the plot located above base ABC represent lithium intercalation compounds. FIG. 2 is a triangular plot representing the plane defined by points A, D and E. The trapezoidal region AEFG in FIG. 2 (but excluding points nearest, e.g., within about 0.01 transition metal mole units(モル単位), to the vertices A and D) illustrates an especially preferred set of compositions containing equimolar amounts of manganese and nickel. This preferred set of compositions can be represented by the formula Lia[COxNi1/2Mn1/2)1-x]O2, where 0≦a≦1.2 and 0.1≦x≦0.98. The compounds of Formulas I, II and III are shown as points within region AEFG."
"Example 1
Metal containing precursors were combined in proportions to yield(を得る、となる比率、割合で混合した)the final oxide composition LiNi0.1Mn0.1Co0.8O2. Accurate batching(回分?)was achieved by assaying(測定、定量?)the precursors. The assays were performed by baking aliquots(アリコート、分割量)of the precursors at 600° C. overnight to yield completely water free single phase oxides. Measurements(測定値)of the weights before and after heating combined with the knowledge of the final phase composition were used to calculate the mass per mole of metal in each precursor. This method allowed batching with at least a ±/−0.1 weight percent precision(精度で). The precursors NiCO3 (22.44 parts, available from Spectrum Chemical) and MnCO3 (21.48 parts, Spectrum Chemical) were placed(置いた)in a 1 liter high-density polyethylene Sweco™ mill jar (available from Sweco) along with(と共に)333 parts Zircoa™ 12.7 mm radius end cylinder zirconium oxide media (available from Zircoa, Inc.) and 1000 parts of similar 6.35 mm ZIRCOA zirconium oxide media. 200 parts deionized (DI) water were added to the mill jar and the nickel and manganese carbonates were wet-milled in a Sweco M18-5 mill (available from Sweco) for 24 hours. Li2CO3 (68.12 parts, available from FMC, Philadelphia, Pa.), Co(OH)2 (137.97 parts, available from Alfa Aesar) and an additional 100 parts DI water were added to the mill jar, then milled for an additional 4 hours. The resulting(得られた)wet-milled slurry was poured into a PYREX™ cake pan (available from Corning, Inc.) and air-dried(空気乾燥)overnight at 70° C. The dried cake was scraped from the pan, separated from the media and granulated(粒状)through a 25 mesh (707 μm) screen. The resulting screened powder was placed in a clean polyethylene bottle and the lid sealed with tape.
15 Parts of the screened powder were placed in an alumina crucible and heated from room temperature to 900° C. in oxygen over a one hour period, held at 900° C. for 3hours, and cooled. The resulting fired powder(焼成)was submitted for XRD analysis using the Rietveld refinement. The observed XRD pattern indicated that the fired powder had a single phase.
The fired powder was used to form a cathode in an electrochemical cell as described above. The electrochemical cell had a capacity of 146 mAh/g. The irreversible first cycle capacity loss was 5% after charging and discharging the cell to 4.3 volts.
US5783333 (Lithium nickel cobalt oxides for positive electrodes)
"Positive electrodes including a lithium nickel cobalt metal oxide are disclosed(開示する). The lithium nickel cobalt metal oxides have the general formula(一般式)Lix Niy COz Mn O2, where M is selected from the group consisting of(からなる群から選択)aluminum, titanium, tungsten, chromium, molybdenum, magnesium, tantalum, silicon, and combinations thereof(それらの組み合わせ), x is between about 0 and about 1 and can be varied within this range by electrochemical insertion and extraction, the sum of y+z+n is about 1, n ranges between above 0 to about(約、およそ)0.25, y and z are both greater than 0, and the ratio z/y ranges from above 0 to about 1/3. Also disclosed are(も開示する)composite(複合)positive electrodes including the abovedescribed(上記)lithium nickel cobalt metal oxides together with(共に)a lithium manganese metal oxide of the formula Lix Mn2-r M1r O4, where r is a value between 0 and 1 and M1 is chromium, titanium, tungsten, nickel, cobalt, iron, tin, zinc, zirconium, silicon, or a combination thereof." (Abstract)
"Due to the increasing demand for(需要が高まっている)battery-powered electronic equipment, there has been a corresponding increase in demand for rechargeable battery cells having high specific energies. In order to meet this demand(需要に対応するため), various types of rechargeable cells have been developed, including improved aqueous nickel-cadmium batteries, various formulations of aqueous nickel-metal hydride batteries and, recently, nonaqueous rechargeable lithium-ion cells (sometimes referred to as "lithium rocking chair," or "lithium intercalation" cells). Lithium-ion cells are particularly attractive(有望、期待、注目、関心を集めている)because they have a high cell voltage and a high specific energy."
"Various positive electrodes ("cathodes" on discharge) have been studied(研究)and/or used in lithium ion batteries. These include lithium molybdenum sulfides(複数形), lithium molybdenum oxides, lithium vanadium oxides, lithium chromium oxides, lithium titanium oxides, lithium tungsten oxides, lithium cobalt oxides, lithium nickel oxides, and lithium manganese oxides. The preparation(調製)and use of lithium transition metal oxide positive electrodes are described in various publications including U.S. Pat. Nos. 4,302,518 and 4,357,215 issued to Goodenough et al., which are incorporated herein by reference for all purposes."
"While these materials, particularly lithium cobalt oxide(無冠詞)(LiCoO2), lithium nickel oxide (LiNiO2), and lithium manganese oxide spinnel (LiMn2 O4), have been found somewhat adequate(ある程度満足), they each have some serious shortcomings(欠点). For example, they may have an unacceptably high irreversible capacity loss. This loss occurs during a first charge cycle when the cell's negative electrode undergoes formation."
""Formation"(フォーメーション、初期充放電)refers to electrode modification processes employed after a cell is assembled, but before it is reversibly cycled; note that some but not all cell types require formation. In cells that require it, formation electrochemically modifies the cell's electrodes so that thereafter they can be reversibly cycled. In lithium ion cells, formation involves(が行われる)an initial cycle which irreversibly drives some lithium ions from the positive electrode material to a carbon negative electrode ("anode" on discharge) where they are believed to(と考えられる)form a surface layer that has been found necessary to provide high energy cycling. This surface layer is known as a solid electrolyte interface or "SEI."
"The ratio of the first cycle charge capacity over the first cycle discharge capacity for a positive electrode is an important parameter in lithium ion cell design. This ratio should be compared to the same ratio for the cell's negative electrode. In all cases, the positive electrode's first cycle capacity ratio should be designed to match the negative electrode's first cycle capacity ratio. If the positive electrode ratio exceeds the negative electrode ratio, lithium metal electroplating can occur, which can result in undesirable capacity fading and safety problems. In the case where(の場合)this ratio is larger for the negative electrode, the cell's reversible capacity is limited by the negative electrode. Likewise(同様に), when the opposite is true(逆の場合), the cell is limited [by] the positive electrode."
"The problem of a positive electrode with a high first cycle ratio can be further understood by considering the example of lithium nickel oxide. As this material has a high first cycle charge ratio, less of it is required to "form" a given(所定の、一定の)amount of carbon negative electrode than is required to reversibly cycle against that same amount of negative electrode (assuming that(仮定して)the negative electrode has a lower first charge ratio). Thus(従って), if a cell is provided with an amount of lithium nickel oxide sufficient for formation, that cell will have insufficient lithium nickel oxide to utilize the available negative electrode material during subsequent reversible cycles. That is(すなわち), the negative electrode will be underutilized, with some fraction of it constituting(なる、構成する)useless mass (which reduces the cell's specific energy). On the other hand, if more lithium nickel oxide is used in the cell (beyond that required for formation), some metallic lithium will electroplate onto(電気めっきする)the negative electrode during formation, presenting the danger that(危険性、リスク)the electroplated lithium metal will undergo(引き起こす、生ずる、晒される)an exothermic chemical reaction."
"By designing a mixed oxide to(~するように設計する)include nickel plus another metal which tends to equalize the amount of oxide required to reversibly cycle against and form a given amount of negative electrode material, the above difficulties can be mitigated(低減、緩和、抑制). Lithium nickel cobalt oxides are potentially useful candidates for such applications because the presence of cobalt does, in fact, tend to equalize the amount of oxide required for these two functions. Note that(ここで、但し), in contrast to lithium nickel oxide, more lithium cobalt oxide is required to form a negative electrode than to reversibly cycle against it. Thus, it intuitively follows that(直観的に分かる)the presence of cobalt in a nickel oxide will tend to match the formation capacity and reversible capacity of the oxide."
US5057692 (High speed, radiation tolerant, CT scintillator system employing garnet structure scintillators)
"This class of scintillator material is based on activated luminescence of cubic garnet crystals. Garnets are a class of materials with the crystal chemical formula A3 B5 O12 in which the A cations are eight-coordinated(8配位)with oxygens and the B cations are either octahedrally (six) or tetrahedrally (four) coordinated(8面体または4面体配位)with oxygens. The crystal structure is cubic with 160 ions per unit cell containing eight formula units. In accordance with the present invention, the A cations are rare earth or yttrium ions alone, in combinations and/or with activator substitutions. The B cations may be rare earth ions or other ions, again, alone, in combinations and/or with substitutions. In particular, we have found that with activator ions substituted in the eight-coordinated or six-coordinated(8配位または6配位)sites, these garnets are luminescent in response to x-ray stimulation. A particularly important activator ion which we have discovered is x-ray luminescent in this host material is the chromium 3+ ion located in six-coordinated(6配位)sites."
US8808807 (Functionalized inorganic films for ion conduction)
"To increase the water retention in the porous inorganic films, hydrophilic species, such as silica, alumina, niobia, or aluminosilica, are incorporated into the inorganic framework along the pore surfaces. The formation of Brønsted acid sites in the framework increases the acidity and hydrophilicity of the material. For example, treatment(処理)of the mesoporous silica film with an alkaline solution containing soluble aluminum species yields(生み出す、生ずる)an aluminosilica film that has four-coordinated(4配位)Al—O—Si sites (e.g., Brønsted or Lewis acid sites) incorporated on the surface of the silica framework. As shown in FIG. 3, the quantity of four-coordinated(4配位)Al sites in the film is determined by single-pulse 27Al MAS NMR. The peaks at 113 ppm, 5 ppm, and 9 ppm correspond to AIN, four-coordinated Al, and six-coordinated(6配位)Al, respectively. About 90% of the incorporated aluminum atoms in the film possess approximately a tetrahedral coordination(4面体配位), while the remaining aluminum atoms have closer to octahedral coordination(8面体配位)corresponding to framework aluminum atoms that are coordinated(配位)additionally with adsorbed water molecules or that are extra-framework species in the form of macroscopically phase-separated Al2O3. Two-dimensional HETeronuclear chemical shift CORrelation (HETCOR) NMR spectroscopy establishes unambiguously that a substantial fraction of the six-coordinated(6配位)Al atoms are interacting strongly with adsorbed water, consistent with the increased hydrophilicity of the film. Correlated signal intensity in the 29Si{1H} HETCOR spectrum in FIG. 4 shows that four-coordinated(4配位)framework 29Si sites are interacting with water protons in the sample. Specifically, cross-peaks associated with chemical shift correlations between the protons of water and the 29Si species that are covalently bonded to framework 27Al species through an oxygen bridge are clearly evident in FIG. 4. The corresponding experimental conditions(実験条件)are: 10 kHz spinning rate, 3.6 μs 90° 1H pulse, 1 s recycle delay, and 2096 scans for each of the 64 t1, increments."
US9194682 (Carbon baking oxygen preheat and heat recovery firing system)
"The inventors have discovered(発見、見出し)that a carbon baking ring furnace can be equipped with a supplemental oxygen conduit that is configured to also allow recycling of waste heat from the cooling zone to the firing and/or preheat zone system to so significantly reduce fuel (e.g., natural gas), in many cases up to 25% to 40% reduction, while at the same time allowing for complete combustion of volatiles and pitch."
US5859362 (Trace vapor detection)
"The inventors have discovered(発見、見出し)that many cocaine seized samples not only emit relatively small amounts of cocaine vapors but, more importantly, emit vapors of ecgonidine methyl ester (EDME), a well known structurally related degradation product of cocaine comprised of a bicyclic skeleton."
US20090082410 (CONSTITUTIVELY TRANSLOCATING CELL LINE)
"The present inventors have discovered(発見、見出し)that GPCRs containing one or more sites of phosphorylation, preferably clusters of phosphorylation sites, properly positioned in its carboxyl-terminal tail have an increased affinity for arrestin and colocalize with arrestin in endosomes upon GPCR phosphorylation, either after stimulation with agonist or in an agonist-independent manner as described herein."
"The twin LIGO instruments ... use a split laser beam to detect infinitesimal changes in length between L-shaped arms as gravitational waves pass through" (重力波発見の記事。The Japan Times, 13SEP2016, page 1)
splitしたんだからbeamsじゃないかという気もする。日本語の原稿で同様の文章を英訳する場合とか、迷いそうだ。しかし、不定冠詞が「と(か)言うもの」、「~なるもの」のような意味だと考えればいいのかもしれない。複数形にすると却って意味を狭めるのかも。しかしこれは新聞の記事であって科学論文では無いし、「厳密にはsplit laser beamsがいい」、というネイティブもいるかもしれない。
後記(30JUNE2017)
単に、split beamsのうち1つを使っているということだろう。
US6630507(Cannabinoids as antioxidants and neuroprotectants)
"EXAMPLE 1 Preparation of Cannabinoids and Neuronal Cultures
Cannabidiol, THC and reactants other than those specifically listed below were purchased from Sigma Chemical, Co. (St. Louis, Mo.). Cyclothiazide, glutamatergic ligands and MK-801 were obtained from Tocris Cookson (UK). Dihydrorhodamine was supplied by Molecular Probes (Eugene, Oreg.). T-butyl hydroperoxide, tetraethylammonium chloride, ferric citrate and sodium dithionite were all purchased from Aldrich (WI). All culture media were Gibco/BRL (MD) products."
US7295492 (Method and apparatus for correlation sonar)
"FIRST COMPARATIVE EXAMPLE
Prior-art method 200 for a velocity-measuring correlation SONAR is applied to receiver array 104. Operations 202 through 214 are performed to determine that the receiver pair (1, 14) has the best correlation. Velocity vector 420 for best-correlated primary receiver pair (1,14) is depicted in FIG. 4. A velocity estimate is developed based on pair (1,14), as per operations 216 and 218.
EXAMPLE APPLYING THE ILLUSTRATIVE METHOD TO THE FIRST COMPARATIVE EXAMPLE
In this Example, the inventive method is applied to the scenario of Comparative Example 1. Instead of developing only a single velocity solution based on the best-correlated primary receiver pair (1, 14) and other primary receiver pairs, a second solution is developed based on a set of redundant receiver pairs that includes a redundant pair that has the same velocity vector as the best-correlated primary receiver pair as well as additional redundant receiver pairs that have the same velocity vector as other primary receiver pairs. Receiver pair (5, 8) is a redundant receiver pair that has the same velocity vector as the best-correlated primary receiver pair. FIG. 5 depicts velocity vector 420 for best-correlated primary receiver pair (1, 14) and velocity vector 520 for corresponding redundant receiver pair (5, 8). Other redundant receiver pairs that correspond to the best-correlated primary receiver pair (1,14) and that could serve as the basis for additional velocity estimates include redundant pairs (16, 13) and (6, 9)."
US7251609 (Method for conducting clinical trials over the internet)
"EXAMPLE I
Osteoarthritis is a Public Health Problem
Osteoarthritis is a common age-related disorder, with radiographic changes present in the knees of over 10% of the population aged over 45 years, and which causes a substantial burden of disability and economic cost in the elderly. Its prevalence in the US is believed to exceed 60 million people, and the incidence of symptomatic knee and hip OA has been estimated at 200/100,000 person years. It is responsible for some 68 million work loss days per year, and for 70% of all hip replacements at a cost of $3 billion, in the US annually."
US6912222 (Private network access point router for interconnecting among internet route providers)
"EXAMPLE 2
Configuration for a Multi-PNAP Customer
In the example below assume the customer is connected to two PNAPs, A and B. A is the primary, with connections to NSP C, NSP D, while B is the secondary, with connections to NSP C, NSP D, and NSP E."
US7855647 (Flame resistant RFID tag and method of making the same)
"Comparative Example 1
A dry polyester (PET) inlay with squiggle antenna pattern (having the trade designation “SQUIGGLE”, available from Alien Technologies, Morgan Hill, Calif., part #1850032-001 supplied in roll form), was tested with no pressure sensitive adhesive layer. This commercial “SQUIGGLE” inlay as supplied includes a thin epoxy adhesive bonding the copper to the polyester. This inlay part number also includes an IC chip. The dimensions of the specimen tested was greater than 2 inches (5 cm)×12 inches (30 cm), cut from a roll of inlays 6 inches (15 cm) wide. The inlay was tested in both antenna orientations in accordance with the Burn Test.
Comparative Example 2
RFID “squiggle” tags were cut from the inlay roll from Comparative Example 1. They were cut in such a way to minimize the amount of PET in the sample. These trimmed inlays was attached to a 0.002 inch (50 micrometers) thick sheet of polyimide film 3 inches (8 cm) by 12 inches (30 cm) (having the trade designation “KAPTON H”, available from E.I. du Pont de Nemours and Co., Wilmington, Del.) using a flame resistant acrylic adhesive transfer tape marketed under the trade designation “9372 DK” by 3M Company, St. Paul, Minn.). The antennas were placed such that they were spaced between each other by 0.25 inch (6 mm). A second layer of 3M Company 9372 DK adhesive transfer tape was then applied over the first adhesive layer to encapsulate the RFID “squiggle” tags. Two Burn Tests were conducted, one with the major axis of the antenna oriented vertical and the other with the major axis of the antenna oriented horizontally
Example 1
An 0.002 inch (50-micrometer) thick sheet of polyimide film (having the trade designation “KAPTON H”, available from DuPont, Wilmington, Del.), was etched to form single dipole copper antennas each occupying an area 3 inches (8 cm) long and 0.75 inch (19 mm) wide, spaced 2 inches (5 cm) apart. A Phillips SL3ICS3001FTT UCODE EPC G2 RFID chip (available from Koninklijke Philips Electronics N.V., Groenewoudseweg 1, 5621 BA, Eindhoven, Netherlands) was attached to the substrate. The polyimide film was cut into strips at least 3 inches (8 cm) wide and 12 inches (30 cm) long. Specimens were prepared with the primary axes of the antenna oriented vertically and horizontally in accordance with the Burn Test. Each sample was laminated with “9372 DK” adhesive transfer tap on the side with the antenna and integrated circuit. The samples were then tested in accordance with the Burn Test."
"Soon after, according to Chen's mother, one of the couple's friends, who had ties to the first bank, told them that it had a policy of refusing loans to residents of the 17-story Weiguan Golden Dragon Building, due to its poor construction" (The Japan Times, 9FEB2016, page 4; about a Taiwan apartment building that collapsed in a quake)
whoの先行詞はone, それともfriends?
多分oneだと思うが自信は無い。whoの前にコンマが無ければfriendsに係る?。
"The blast blew a hole in the side of a Daallo Airlines jet" (The Japan Times, 9FEB2016, page 5; caption to a photo of damaged aircraft)
(厳密には胴体の一(右)側面だが、定冠詞でthe side。「側面」という情報だけ伝えたいということだろうが、特許翻訳で使うには相当自信が要る)
(追記23JUNE2022)意味からすればoneのはず。"it had a policy of refusing loans"という情報を持っているのはfirst bankに詳しい者のはずだから、them(friends?)にその情報を言ったのはone of the couple's friends。
しかし音で聞いた場合を想像すると、先行詞はfriendsかと(私なら)思いそう。
(グーグル翻訳)
修正原稿:According to Chen's mother, one of the couple's friends, who had ties to the bank, told them that it had a policy of refusing loans to the residents of the building
グーグル翻訳:「陳氏の母親によると、銀行と関係のある夫婦の友人の一人は、建物の住民への融資を拒否する方針を持っていると彼らに言った」(銀行と関係があるのは誰か不明瞭)
US7204894 (Annealing of hot rolled steel coils with clam shell furnace)
"In this type of high temperature annealing, the use of a normal air atmosphere(通常の空気(大気?)雰囲気)during the annealing promotes decarburization of the steel coil. The combination of high heat and a normal air atmosphere initially depletes a layer of carbon on the surface of the steel coil and decarburization results."
US4079156 (Conductive metal pigments)
"Conductive metal pigments which may be used in electrical devices are prepared by forming an alloy of a non-noble conductive metal and at least one oxidizable material, mixing the resulting alloy with a vitreous frit and an organic vehicle to form an ink, screening said ink onto a substrate, firing the alloy in an air atmosphere(空気雰囲気)at a temperature in excess of about 500° C. and cooling the ink to produce a conductive pigment."
US7368409 (Regeneration method of heterogeneous catalysts and adsorbents)
"This operation of regeneration has often to be preceded of stripping step consisting of heating step, either under air or inert atmosphere(空気または不活性雰囲気)(nitrogen or lean gas) in order to eliminate some free hydrocarbons contained in the catalyst porosity, just by promoting a phenomenon of evaporation."
「大気雰囲気」はan atmospheric atmosphere? the atmosphere? an air atmosphere?
atmospheric atmosphereとの記載はググってもネイティブの英語ではほとんど見つからない。
追記11FEB2016
WO 2013161969 A1 ゴム成形体の製造方法(ニチアス株式会社)
「大気雰囲気下とは、酸素を含有する大気雰囲気下を示すこととする。すなわち、従来架橋剤として用いられている過酸化物に由来するCOOラジカル(COO・)が失活する大気雰囲気下を示すこととする。例えば、本発明における大気雰囲気下は、空気雰囲気下であることとしてもよいし、窒素約80vol%、酸素約20vol%を含有する大気雰囲気下であることとしてもよいし、酸素の体積含有率が10vol%以上、3vol%以上、あるいは1vol%以上の大気雰囲気下であることとしてもよい。」(段落0091)
US2015/0137408 (上記の対応US出願;日本語からの翻訳)
"[0088] In addition, the atmospheric atmosphere in the present invention refers to an atmospheric atmosphere containing oxygen. That is, the atmospheric atmosphere refers to an atmospheric atmosphere in which a COO radical (COO.) derived from a peroxide, which has heretofore been used as a crosslinking agent, is deactivated. For example, the atmospheric atmosphere in the present invention may be an air atmosphere, may be an atmospheric atmosphere containing about 80 vol % of nitrogen and about 20 vol % of oxygen, or may be an atmospheric atmosphere having an oxygen volume content of 10 vol % or more, 3 vol % or more, or 1 vol % or more."
数少ないネイティブ英語の例:
US4717798 (Low voltage vacuum circuit interrupter)
"4. The vacuum circuit interrupter of claim 3 wherein said enclosed space is at least partially evacuated for returning said metal diaphragm and said contact to said first position by difference in pressure between atmospheric atmosphere and reduced pressure within said enclosed space when said control signal is removed from said spaced wires."
US3460818 (Apparatus for treatment of particulate material on moving support)
"The downdraft gas flow through the grate and balls in the induration zone arises from a super-atmospheric atmosphere, such as about 10 inches of water, maintained above the layer of balls by conduit 74, and from a suitable subatmospheric pressure maintained below the layer of balls in the indurating zone by suction of fans 79."
空気雰囲気(上にあるが)も少ないような気がする。
US5086016 (Reservoir feed method of making ceramic composite structures and structures made thereby)
"EXAMPLE 3
A permeable preform of the shape of preform 56 in FIG. 5 was prepared by the technique of Table A, steps (B) and (C), using the same sedimentation casting composition except that only 5 parts by weight of the silicon metal powder was used. The preform was coated on its outer surface with two thin layers of air-permeable plaster of paris barrier material and the same aluminum alloy as in Table C was provided as the parent metal in a vessel (58 in FIG. 5). The lay-up was heated in an air atmosphere for 68 hours at 1000° C."
US20040009359 A1 (Alpha Al2O3 and Ti2O3 protective coatings on aluminide substrates)
"The process includes creating a dry air atmosphere with a concentration of water vapor below about 750 ppm at a temperature above about 550° C."
US8439108 (Application of degradable polymers in sand control)
"Further development(開発)is underway for other degradable or biodegradable polymers. Metabolix, Inc. of Cambridge, Mass., for example, is developing a family of degradable polymers known as PHAs (polyhydroxyalkanoates). PHA polymers (also polyesters) are produced by photosynthesis, either indirectly using highly efficient fermentation processes, or directly in plant crops."
US8442558 (Detecting, identifying, reporting and discouraging unsafe device use within a vehicle or other transport)
"For example, the Society of Automotive Engineers (SAE) J1939 and J1708 standards are currently in use for heavy duty vehicles, while development is underway for HDOBD (heavy duty OBD) and wireless OBD."
US6191594 (Adapter for a measurement test probe)
"Current development is underway for IC packages having 0.25 mm or 0.01 inches lead spacing."
US8505308 (Integrated direct drive starter/generator for turbines)
"A new, unique podded propulsor concept is being developed that allows optimization of each element of the system."
US5622658 (Water-dispersible granules)
"A relatively new concept is water dispersible granules (also known as dry flowables). These are granular formulations of agricultural chemicals that (when properly formulated), disperse readily in water and remain in suspension, i.e. perform as well as liquid flowables and wettable powders when prepared for spray application to soil or plants. This concept is being developed rapidly by agricultural chemical formulators especially in the U.S.A. and Europe."
1)"[Mr. A], 50, a masseuse who was visiting the monastery for the second time, said, 'Since I started this seated meditation, I have learned to be able to focus on my work'" (The Japan Times, Feb. 1, 2016, page 3、ドイツ人の女性禅僧の記事より;主語修正)
2)"[Mr. A], 50, a masseuse, who was visiting the monastery for the second time, said..."とすると間違い?
1)の場合、初出だから限定用法でいいように感じるが、どうも安心できない、文法的にうまく説明できない。2)が間違いとは断言できない。
他人の英訳チェックをやっていて時々出くわすパターン。
3)"According to the present invention, a control device, which controls the motion of vehicle, comprises ..."
3’)「本発明によれば、制御装置、あ、ついでに言っとくとコイツは車両の動きを制御するんだけど、は・・・を有している」
限定用法:数あるうちの1つに限定;初出の情報に使う、等。
非限定用法:既出の名詞に情報を付加する;当たり前のこと;重要でないこと;除いても文章は成り立つ、等。
1’)「(数多のマッサージ師の中でも)この寺を2度目に訪問していたマッサージ師のAさん50歳はこう語る・・・」
2’)「マッサージ師のAさん50歳、ところでこの寺を訪問するのは2度目なんだが、はこう語る・・・」
どちらでもいいように感じる。どうもよく分からない・・・。















