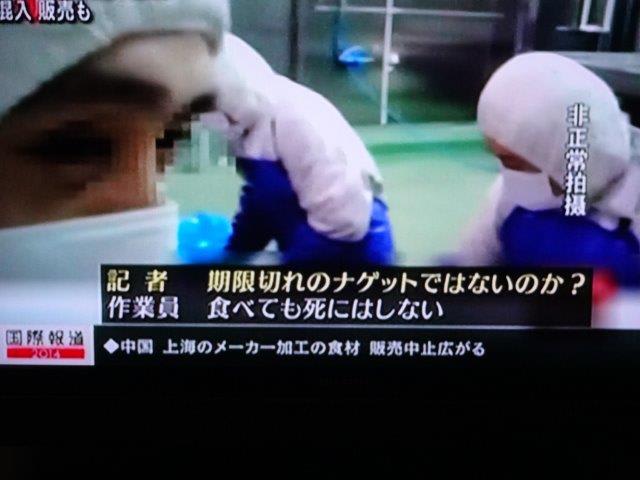
FDAのペットジャーキの追加レポートです。
残念ながら、FDAはまだ特異的原因を特定できていない。
2014年5月1日現在、4,800以上の苦情を受けた。
ほとんどが中国から輸入されたものである。
詳細は下記URL参照願います。
http://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm397713.htm
FDA Provides Latest Information on Jerky Pet Treat Investigation
May 16, 2014
Media Inquiries: Siobhan DeLancey, 202-510-4177, siobhan.delancey@fda.hhs.gov
Trade Media Inquiries: Megan Bensette, 240-506-6818, megan.bensette@fda.hhs.gov
Consumer and Industry Inquiries: AskCVM@fda.hhs.gov
The U.S. Food and Drug Administration is providing an update on its ongoing investigation into pet illnesses and deaths in animals that ate jerky pet treats. This update includes the latest information about complaints of illnesses, FDA’s collaboration with the CDC on a new case control study, and new findings revealed through the agency’s testing. Unfortunately, FDA has still not been able to identify a specific cause for the reported illnesses or deaths.
•Case numbers: Since FDA’s last update on October 22, 2013, we have received approximately 1,800 additional case reports. As of May 1, 2014, we have received in total more than 4,800 complaints of illness in pets that ate chicken, duck, or sweet potato jerky treats, nearly all of which are imported from China. The reports involve more than 5,600 dogs, 24 cats, three people, and include more than 1,000 canine deaths. The breakdown of symptoms associated with the cases is similar to that of earlier reports: approximately 60 percent of the cases report gastrointestinal/liver disease, 30 percent kidney or urinary disease, with the remaining 10 percent of complaints including various other signs such as neurologic, dermatologic, and immunologic symptoms. About 15 percent of the kidney or urinary cases also tested positive for Fanconi syndrome, a rare kidney disease that has been associated with this investigation.
•Response to Dear Veterinarian Letter: Following an October 2013 request for veterinarians to share case information, the agency received many well-documented case reports that have and continue to provide us with valuable information that is assisting in our ongoing investigation. Out of this effort, FDA has had the opportunity to perform necropsies (post-mortem examinations) on 26 dogs, 13 of which appeared to have causes of death not related to consumption of jerky pet treats. Of the remaining 13 cases, an association with consumption of jerky pet treats could not be ruled out. Eleven of these dogs had indications of kidney disease and two involved gastrointestinal disease.
The agency continues to review case records, test treat samples from reported cases, screen tissue, blood, urinary and fecal samples, and communicate with the attending veterinarians and pet owners to thoroughly investigate select cases. Because of the volume of information received in response to the Dear Veterinarian letter, the agency has not completed an update to our online case spreadsheets. FDA plans to complete and post these updates in the coming months.
•Partnership with CDC: While the Centers for Disease Control and Prevention primarily tracks cases of human illness, FDA has requested their expertise in collaborating on a study of cases reported to the FDA of sick dogs compared with “controls” (dogs who have not been ill). The goal of the study is to compare the foods eaten by the sick dogs (cases) to those eaten by the dogs that did not get sick (controls), in order to determine whether sick dogs are eating more jerky pet treats than healthy dogs are. Data collected during this investigation will allow federal investigators to better understand what is making pets sick. The study is still ongoing, and FDA will share results when they are completed.
•Testing: Following testing performed by the New York State Department of Markets and Agriculture (NYSDAM) in 2012 that detected low levels of antibiotics in tested jerky pet treats, FDA undertook a project to adapt the NYSDAM method to the equipment in its own field laboratories for regulatory and enforcement purposes. This adaptation is now complete and the method is in use for testing both imported and domestic treats.
Testing of jerky pet treats from China has also revealed the presence of the drug amantadine in some samples containing chicken. These samples were from jerky pet treats that were sold a year or more ago. Amantadine is an antiviral that is FDA-approved for use in people. It has also been used in an extra-label manner (using an approved drug in a way that isn’t listed on the label) in dogs for pain control, but FDA prohibited its use in poultry in 2006.
FDA does not believe that amantadine contributed to the illnesses because the known side effects or adverse events associated with amantadine do not seem to correlate with the symptoms seen in the jerky pet treat-related cases. However, amantadine should not be present at all in jerky pet treats, and the agency has notified the Chinese authorities that the presence of amantadine in these products is an adulterant. Chinese authorities have also assured us that they will perform additional screening and will follow up with jerky pet treat manufacturers. FDA has notified the U.S. companies that market jerky pet treats found positive for amantadine of this finding and are testing both imported and domestic jerky pet treats for amantadine and other antivirals.
The agency continues to caution pet owners that jerky pet treats are not required for a balanced diet, and encourage them to consult with their veterinarians, both prior to feeding treats and if they notice symptoms in their pets.
FDA continues to devote significant resources to this investigation and to work with its Vet-LIRN partners to gather and analyze new information as it becomes available. If your pet has experienced signs of illness that you suspect is related to jerky pet treats, please report it to FDA. While FDA does not necessarily respond to every individual complaint submitted, each report is valuable and becomes part of the body of knowledge that helps to inform our investigation.
残念ながら、FDAはまだ特異的原因を特定できていない。
2014年5月1日現在、4,800以上の苦情を受けた。
ほとんどが中国から輸入されたものである。
詳細は下記URL参照願います。
http://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm397713.htm
FDA Provides Latest Information on Jerky Pet Treat Investigation
May 16, 2014
Media Inquiries: Siobhan DeLancey, 202-510-4177, siobhan.delancey@fda.hhs.gov
Trade Media Inquiries: Megan Bensette, 240-506-6818, megan.bensette@fda.hhs.gov
Consumer and Industry Inquiries: AskCVM@fda.hhs.gov
The U.S. Food and Drug Administration is providing an update on its ongoing investigation into pet illnesses and deaths in animals that ate jerky pet treats. This update includes the latest information about complaints of illnesses, FDA’s collaboration with the CDC on a new case control study, and new findings revealed through the agency’s testing. Unfortunately, FDA has still not been able to identify a specific cause for the reported illnesses or deaths.
•Case numbers: Since FDA’s last update on October 22, 2013, we have received approximately 1,800 additional case reports. As of May 1, 2014, we have received in total more than 4,800 complaints of illness in pets that ate chicken, duck, or sweet potato jerky treats, nearly all of which are imported from China. The reports involve more than 5,600 dogs, 24 cats, three people, and include more than 1,000 canine deaths. The breakdown of symptoms associated with the cases is similar to that of earlier reports: approximately 60 percent of the cases report gastrointestinal/liver disease, 30 percent kidney or urinary disease, with the remaining 10 percent of complaints including various other signs such as neurologic, dermatologic, and immunologic symptoms. About 15 percent of the kidney or urinary cases also tested positive for Fanconi syndrome, a rare kidney disease that has been associated with this investigation.
•Response to Dear Veterinarian Letter: Following an October 2013 request for veterinarians to share case information, the agency received many well-documented case reports that have and continue to provide us with valuable information that is assisting in our ongoing investigation. Out of this effort, FDA has had the opportunity to perform necropsies (post-mortem examinations) on 26 dogs, 13 of which appeared to have causes of death not related to consumption of jerky pet treats. Of the remaining 13 cases, an association with consumption of jerky pet treats could not be ruled out. Eleven of these dogs had indications of kidney disease and two involved gastrointestinal disease.
The agency continues to review case records, test treat samples from reported cases, screen tissue, blood, urinary and fecal samples, and communicate with the attending veterinarians and pet owners to thoroughly investigate select cases. Because of the volume of information received in response to the Dear Veterinarian letter, the agency has not completed an update to our online case spreadsheets. FDA plans to complete and post these updates in the coming months.
•Partnership with CDC: While the Centers for Disease Control and Prevention primarily tracks cases of human illness, FDA has requested their expertise in collaborating on a study of cases reported to the FDA of sick dogs compared with “controls” (dogs who have not been ill). The goal of the study is to compare the foods eaten by the sick dogs (cases) to those eaten by the dogs that did not get sick (controls), in order to determine whether sick dogs are eating more jerky pet treats than healthy dogs are. Data collected during this investigation will allow federal investigators to better understand what is making pets sick. The study is still ongoing, and FDA will share results when they are completed.
•Testing: Following testing performed by the New York State Department of Markets and Agriculture (NYSDAM) in 2012 that detected low levels of antibiotics in tested jerky pet treats, FDA undertook a project to adapt the NYSDAM method to the equipment in its own field laboratories for regulatory and enforcement purposes. This adaptation is now complete and the method is in use for testing both imported and domestic treats.
Testing of jerky pet treats from China has also revealed the presence of the drug amantadine in some samples containing chicken. These samples were from jerky pet treats that were sold a year or more ago. Amantadine is an antiviral that is FDA-approved for use in people. It has also been used in an extra-label manner (using an approved drug in a way that isn’t listed on the label) in dogs for pain control, but FDA prohibited its use in poultry in 2006.
FDA does not believe that amantadine contributed to the illnesses because the known side effects or adverse events associated with amantadine do not seem to correlate with the symptoms seen in the jerky pet treat-related cases. However, amantadine should not be present at all in jerky pet treats, and the agency has notified the Chinese authorities that the presence of amantadine in these products is an adulterant. Chinese authorities have also assured us that they will perform additional screening and will follow up with jerky pet treat manufacturers. FDA has notified the U.S. companies that market jerky pet treats found positive for amantadine of this finding and are testing both imported and domestic jerky pet treats for amantadine and other antivirals.
The agency continues to caution pet owners that jerky pet treats are not required for a balanced diet, and encourage them to consult with their veterinarians, both prior to feeding treats and if they notice symptoms in their pets.
FDA continues to devote significant resources to this investigation and to work with its Vet-LIRN partners to gather and analyze new information as it becomes available. If your pet has experienced signs of illness that you suspect is related to jerky pet treats, please report it to FDA. While FDA does not necessarily respond to every individual complaint submitted, each report is valuable and becomes part of the body of knowledge that helps to inform our investigation.