とっても退屈な話で申し訳ない。
現役時少し関わった製品(物質)の取り扱いが米国で変わっているのを目にした。
トリクロサン Trichlosanと言う物質があり、名前の通り3つ(tri)の塩素(chlorine)を有し、優れた殺菌効果がある。
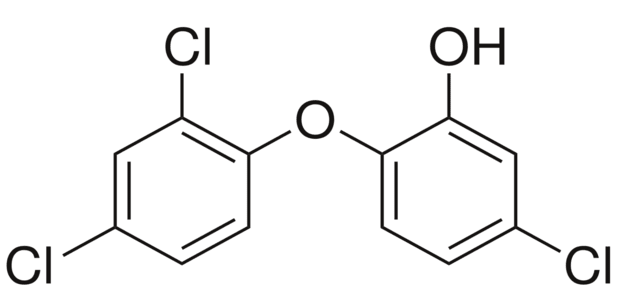
5-chloro-2-(2,4-dichlorophenoxy)phenol
アメリカでは、この優れた殺菌作用のため2,000種以上もの石鹸の他に、歯磨き粉や掃除用洗剤、プラスチック製品や化粧品などにも使用されてきた。
ところがFDA(アメリカ食品医薬品局)は、トリクロサンなど19種類の殺菌剤を含有する抗菌石鹸は、通常の石鹸と比べて優れた殺菌効果があるとは言えない上、免疫系に打撃を与えるリスクがあることを発表し、2017年9月に一般用抗菌石鹸の販売を禁止。
英語版は
The U.S. Food and Drug Administration today issued a final rule establishing that over-the-counter (OTC) consumer antiseptic wash products containing certain active ingredients can no longer be marketed. Companies will no longer be able to market antibacterial washes with these ingredients because manufacturers did not demonstrate that the ingredients are both safe for long-term daily use and more effective than plain soap and water in preventing illness and the spread of certain infections. Some manufacturers have already started removing these ingredients from their products.
This final rule applies to consumer antiseptic wash products containing one or more of 19 specific active ingredients, including the most commonly used ingredients – triclosan and triclocarban. These products are intended for use with water, and are rinsed off after use. This rule does not affect consumer hand “sanitizers” or wipes, or antibacterial products used in health care settings.
“Consumers may think antibacterial washes are more effective at preventing the spread of germs, but we have no scientific evidence that they are any better than plain soap and water,” said Janet Woodcock, M.D., director of the FDA’s Center for Drug Evaluation and Research (CDER). “In fact, some data suggests that antibacterial ingredients may do more harm than good over the long-term.”
The agency issued a proposed rule in 2013 after some data suggested that long-term exposure to certain active ingredients used in antibacterial products — for example, triclosan (liquid soaps) and triclocarban (bar soaps) — could pose health risks, such as bacterial resistance or hormonal effects. Under the proposed rule, manufacturers were required to provide the agency with additional data on the safety and effectiveness of certain ingredients used in over-the-counter consumer antibacterial washes if they wanted to continue marketing antibacterial products containing those ingredients. This included data from clinical studies demonstrating that these products were superior to non-antibacterial washes in preventing human illness or reducing infection.
Antibacterial hand and body wash manufacturers did not provide the necessary data to establish safety and effectiveness for the 19 active ingredients addressed in this final rulemaking. For these ingredients, either no additional data were submitted or the data and information that were submitted were not sufficient for the agency to find that these ingredients are Generally Recognized as Safe and Effective (GRAS/GRAE). In response to comments submitted by industry, the FDA has deferred rulemaking for one year on three additional ingredients used in consumer wash products – benzalkonium chloride, benzethonium chloride and chloroxylenol (PCMX) – to allow for the development and submission of new safety and effectiveness data for these ingredients. Consumer antibacterial washes containing these specific ingredients may be marketed during this time while data are being collected.
Washing with plain soap and running water remains one of the most important steps consumers can take to avoid getting sick and to prevent spreading germs to others. If soap and water are not available and a consumer uses hand sanitizer instead, the U.S. Centers for Disease Control and Prevention (CDC) recommends that it be an alcohol-based hand sanitizer that contains at least 60 percent alcohol.
Since the FDA’s proposed rulemaking in 2013, manufacturers already started phasing out.
殺菌剤メーカーはFDAから求められた「石鹸水洗いと比較して、どれだけ効果があり安全であるか」のデータを提出することができなかった。うーん、それは無理なデーターであるのは理解できる。石鹸をつけて水洗いすれば雑菌は落ちているのに、殺菌剤がさらに必要かと問われると返答に困るということだ。
コロナ対策として石鹸と水でしっかり洗ったあとに、さらにアルコール消毒液をかけるのはあまり意味がない。お店などでアルコール消毒液を手にかけるのは、他人が触れた買い物カゴからの感染防止には効果あるかもしれないが、おばさん達がリンゴやトマトを一つ一つ触っては品定めしているのは止めてくれないか?
日本ではトリクロサンを自然環境ではあり得ない特殊条件下(実験室)で下記の最強毒性ダイオキシンを合成した科学者が論文を発表した経緯もあり使用されていない。

あまりにも退屈すぎる話で申し訳なかったが、私は個人的にアメリカ旅行の際には必ずトリクロサン入り歯磨き剤(コルゲート製)をまとめて買って帰った。もう買えなくなったのかな?それとも歯磨き剤での殺菌効果は認められ製造販売は継続されているのかも?